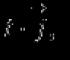Where boilie mariotte law applies. Gas laws
The Boyle-Mariotte law is as follows:
In mathematical form, this statement is written in the form of the formula
where - gas pressure; - gas volume, and - constant value under the agreed conditions. In general, the value is determined by the chemical nature, mass and temperature of the gas.
Obviously, if the index 1 denote the quantities related to the initial state of the gas, and the subscript 2 - to the final, then the given formula can be written in the form
.
From the above and the above formulas, the form of the dependence of the gas pressure on its volume in the isothermal process follows:
This dependence is another, equivalent to the first, expression of the content of the Boyle - Mariotte law. It means that
The pressure of a mass of gas at a constant temperature is inversely proportional to its volume.
Then the relationship between the initial and final states of the gas participating in the isothermal process can be expressed as:
It should be noted that the applicability of this and the above formula, which connects the initial and final pressures and volumes of gas with each other, is not limited to the case of isothermal processes. The formulas remain valid in those cases when the temperature changes during the process, but as a result of the process, the final temperature turns out to be equal to the initial one.
It is important to clarify that this law is valid only in cases where the gas in question can be considered ideal. In particular, the Boyle - Mariotte law is fulfilled with high accuracy in relation to rarefied gases. If the gas is strongly compressed, then significant deviations from this law are observed.
Consequences
Boyle's Law - Mariotte states that the pressure of a gas in an isothermal process is inversely proportional to the volume occupied by the gas. If we take into account that the density of a gas is also inversely proportional to the volume it occupies, then we come to the conclusion:
In an isothermal process, the gas pressure changes in direct proportion to its density.
Thus, we come to the conclusion:
The isothermal coefficient of compressibility of an ideal gas is equal to the reciprocal of its pressure.
see also
Write a review about Boyle's Law - Mariotte
Notes (edit)
- Petrushevsky F.F.// Encyclopedic Dictionary of Brockhaus and Efron
- // Physical encyclopedia / Ch. ed. A.M. Prokhorov. - M .: Soviet Encyclopedia, 1988 .-- T. 1. - S. 221-222. - 704 p. - 100,000 copies
- Sivukhin D.V. General course of physics. - Moscow: Fizmatlit, 2005 .-- T. II. Thermodynamics and Molecular Physics. - S. 21-22. - 544 p. - ISBN 5-9221-0601-5.
- Elementary textbook of physics / Ed. G. S. Landsberg. - M .: Science, 1985. - T. I. Mechanics. Heat. Molecular physics. - S. 430 .-- 608 p.
- Kikoin A.K., Kikoin I.K. Molecular physics. - M .: Nauka, 1976 .-- S. 35-36.
- At constant mass.
- Livshits L. D.// Physical encyclopedia / Ch. ed. A.M. Prokhorov. - M .: Great Russian Encyclopedia, 1994 .-- T. 4. - S. 492-493. - 704 p. - 40,000 copies - ISBN 5-85270-087-8.
Literature
- Petrushevsky F.F.// Encyclopedic Dictionary of Brockhaus and Efron: in 86 volumes (82 volumes and 4 additional). - SPb. , 1890-1907.
Excerpt from Boyle's Law - Mariotte
“She's the one,” a rough woman’s voice was heard in response, and after that Marya Dmitrievna entered the room.All the young ladies and even the ladies, except for the oldest, stood up. Marya Dmitrievna stopped in the doorway and, from the height of her corpulent body, holding her fifty-year-old head high with gray curls, looked at the guests and, as if rolling up, unhurriedly straightened the wide sleeves of her dress. Marya Dmitrievna always spoke Russian.
“Dear birthday girl with the children,” she said in her loud, thick voice overwhelming all other sounds. - What are you, an old sinner, - she turned to the count, who kissed her hand, - tea, do you miss Moscow? There is nowhere to chase the dogs? But what, father, to do, this is how these birds grow up ... - She pointed to the girls. - If you want or not, you have to look for suitors.
- Well, what, my Cossack? (Marya Dmitrievna called Natasha a Cossack) - she said, caressing Natasha with her hand, who approached her hand without fear and cheerfully. - I know that the potion is a girl, but I love it.
She took out yacht earrings with pears from a huge reticule and, giving them to Natasha, who was shining and blushing for her birthday, immediately turned away from her and turned to Pierre.
- Eh, eh! kind! come here, ”she said in a feigned low and thin voice. - Come on, my dear ...
And she rolled up her sleeves menacingly.
Pierre approached, looking naively at her through his glasses.
- Come, come, dear! I told your father the truth alone, when he was in the case, and then God commands you.
She paused. Everyone was silent, expecting what would happen, and feeling that there was only a preface.
- Good, there is nothing to say! good boy! ... Father lies on the bed, and he amuses himself, puts the quartermaster on a bear. Ashamed, father, ashamed! It would be better if he went to war.
She turned away and held out her hand to the count, who could hardly refrain from laughing.
- Well, well, to the table, I have tea, is it time? - said Marya Dmitrievna.
The count went ahead with Marya Dmitrievna; then the countess, who was led by the hussar colonel, the right person with whom Nikolai had to catch up with the regiment. Anna Mikhailovna - with Shinshin. Berg gave his hand to Vera. Smiling Julie Karagina went with Nikolai to the table. Other couples followed them, stretching across the hall, and behind them all, one by one, children, tutors and governesses. The waiters stirred, the chairs rattled, music played in the choirs, and the guests settled down. The sounds of the count's home music were replaced by the sounds of knives and forks, the guests talking, the quiet steps of the waiters.
The Countess sat at one end of the table. On the right is Marya Dmitrievna, on the left is Anna Mikhailovna and other guests. At the other end sat the count, on the left a hussar colonel, on the right Shinshin and other male guests. On one side of the long table there are older youth: Vera next to Berg, Pierre next to Boris; on the other hand, children, tutors and governesses. From behind the crystals, bottles and vases of fruit, the count looked at his wife and her high cap with blue ribbons and diligently poured wine to his neighbors, not forgetting himself. The countess also, because of the pineapples, not forgetting the duties of the hostess, threw significant glances at her husband, whose bald head and face, it seemed to her, were more sharply different from the gray hair in their redness. There was an even babbling on the ladies' end; on the men's one, voices were heard louder and louder, especially of the hussar colonel, who ate and drank so much, blushing more and more that the count was already setting him up as an example to other guests. Berg spoke with Vera with a gentle smile that love is not an earthly feeling, but a heavenly one. Boris called his new friend Pierre the guests at the table and exchanged glances with Natasha, who was sitting opposite him. Pierre spoke little, looked around at new faces and ate a lot. Starting from two soups, of which he chose a la tortue, [tortoiseshell,] and kulebyaki, to hazel grouses, he did not miss a single dish and not a single wine, which the butler mysteriously poked out of a neighbor's shoulder in a bottle wrapped in a napkin, saying or “dray Madeira, or Hungarian, or Rhine. He substituted the first of the four crystal glasses with the count's monogram, standing in front of each device, and drank with pleasure, glancing more and more pleasantly at the guests. Natasha, sitting opposite him, looked at Boris as girls of thirteen look at the boy with whom they had just kissed for the first time and with whom they are in love. This very gaze of her sometimes turned to Pierre, and under the gaze of this funny, lively girl he wanted to laugh himself, not knowing why.
Nikolay was sitting far from Sonya, next to Julie Karagina, and again with the same involuntary smile he was talking to her. Sonya smiled ceremoniously, but, apparently, was tormented by jealousy: she turned pale, then blushed and with all her might listened to what Nikolai and Julie were saying among themselves. The governess looked around uneasily, as if preparing for a rebuff, if anyone had thought to offend the children. The German governor tried to memorize all kinds of food, desserts and wines in order to describe everything in detail in a letter to his family in Germany, and was very offended that the butler, with a bottle wrapped in a napkin, carried him around. The German frowned, trying to pretend that he did not want to get this wine, but was offended because no one wanted to understand that he needed wine not to quench his thirst, not out of greed, but out of conscientious curiosity.
At the male end of the table, the conversation grew more and more lively. The colonel said that the manifesto on the declaration of war had already been published in St. Petersburg and that the copy he had seen himself had now been delivered by courier to the commander-in-chief.
22. Boyle-Mariotte's Law
One of the laws of ideal gas is Boyle-Mariotte's law, which reads: the product of pressure P by volume V gas at constant gas mass and temperature constant. This equality is called isotherm equations... The isotherm is depicted on the PV-diagram of the gas state in the form of a hyperbola and, depending on the gas temperature, occupies one or another position. The process taking place at T= const is called isothermal. Gas at T= const has constant internal energy U. If the gas expands isothermally, then all the heat goes to work. The work that the gas does, expanding isothermally, is equal to the amount of heat that must be imparted to the gas to perform it:
dА= dQ= PdV,
where d A- elementary work;
dV- elementary volume;
P- pressure. If V 1> V 2 and P 1< P 2 , то газ сжимается, и работа принимает отрицательное значение. Для того чтобы условие T= const was fulfilled, it is necessary to consider the changes in pressure and volume as infinitely slow. There is also a requirement for the medium in which the gas is located: it must have a sufficiently high heat capacity. Calculation formulas are also suitable in the case of supplying thermal energy to the system. Compressibility gas is called its property to change in volume when the pressure changes. Every substance has compressibility factor, and it is equal to:
c = 1 / VО (dV / CP) T,
here the derivative is taken at T= const.
The compressibility factor is introduced to characterize the change in volume with a change in pressure. For an ideal gas, it is equal to:
c = -1 / P.
In SI, the compressibility factor has the following dimension: [c] = m 2 / N.
This text is an introductory fragment. From the book Creativity as an Exact Science [Theory of Inventive Problem Solving] the author Altshuller Genrikh Saulovich1. The law of completeness of parts of a system A necessary condition for the fundamental viability of a technical system is the presence and minimum performance of the main parts of the system. Each technical system must include four main parts: the engine,
From the book Interface: New Directions in the Design of Computer Systems author Ruskin Jeff2. The law of "energy conductivity" of a system A necessary condition for the fundamental viability of a technical system is the through passage of energy through all parts of the system. Any technical system is an energy converter. Hence the obvious
From the book Instrumentation author Babaev MA6. The law of transition to the supersystem Having exhausted the possibilities of development, the system is included in the supersystem as one of its parts; in this case, further development takes place at the level of the supersystem. We have already spoken about this law. Let's move on to the "dynamics". It includes laws reflecting
From the book Heat engineer the author Burkhanova Natalia7. The law of transition from the macrolevel to the microlevel The development of the working organs of the system goes first to the macro - and then to the micro level. In most modern technical systems, the working bodies are "pieces of iron", for example, aircraft propellers, car wheels, cutters
From the book Computational Linguistics for All: Myths. Algorithms. Language the author Anisimov Anatoly Vasilievich8. The law of increasing the degree of su-field Development of technical systems goes in the direction of increasing the degree of su-field. The meaning of this law lies in the fact that non-field systems tend to become su-field, and in su-field systems, development goes in the direction
From the book The Phenomenon of Science [Cybernetic Approach to Evolution] the author Turchin Valentin Fedorovich From the book Nanotechnology [Science, Innovation and Opportunity] by Foster Lynn4.4.1. Fitts's Law Imagine that you move the cursor to a button on the screen. The button is the target of this movement. The length of the straight line connecting the start position of the cursor and the nearest point of the target is defined in Fitts's Law as a distance. On
From the book History of Outstanding Discoveries and Inventions (Electrical Engineering, Electric Power Engineering, Radio Electronics) the author Schneiberg Jan Abramovich4.4.2. Hick's Law Before moving the cursor to a target or performing any other action from a set of many options, the user must select this object or action. Hick's Law states that when it is necessary to make a choice from n options, the time to choose
From the author's book9. Poisson and Gauss distribution law Poisson's law. Its other name is the law of ra-definition of rare events. Poisson's law (Z.P) is applied in cases where it is unlikely, and therefore the use of B / Z / R is impractical. The advantages of the law are: convenience with
From the author's book23. Gay-Lussac's law Gay-Lussac's law says: the ratio of the volume of a gas to its temperature at constant gas pressure and mass is constant. V / T = m / MO R / P = const at P = const, m = const. This equality is the name of the isobar equation. The isobar is depicted on the PV-diagram of a straight line,
From the author's book24. Charles's law Charles's law states that the ratio of gas pressure to its temperature is constant if the volume and mass of the gas are unchanged: P / T = m / MO R / V = const at V = const, m = const. This equality is called the isochore equation .Isochora is depicted on the PV-diagram by a straight line parallel to the P axis, and
From the author's book30. The law of conservation and transformation of energy The first law of thermodynamics is based on the universal law of conservation and transformation of energy, which states that energy is not created and does not disappear. Bodies participating in the thermodynamic process interact with each other
From the author's bookTSAREVNA-FROG AND THE LAW OF STABILITY As already emphasized earlier (the law of abstraction), primitive thinking skillfully analyze concrete phenomena and synthesize new abstract systems. Since any object constructed by consciousness was perceived as living, and living
From the author's book1.1. The Basic Law of Evolution In the process of the evolution of life, as far as we know, there has always been an increase in the total mass of living matter and the complication of its organization. By complicating the organization of biological formations, nature acts by the method of samples and
From the author's book4.2. Moore's Law In its simplest form, Moore's Law boils down to stating that the wiring density of transistor circuits doubles every 18 months. The authorship of the law is attributed to one of the founders of the well-known Intel company, Gordon Moore. Strictly speaking, in
DEFINITION
The processes in which one of the parameters of the gas state remains constant are called isoprocesses.
DEFINITION
Gas laws are the laws describing isoprocesses in an ideal gas.
Gas laws were discovered experimentally, but they can all be obtained from the Mendeleev-Clapeyron equation.
Let's consider each of them.
Boyle-Mariotte's law (isothermal process)
Isothermal process is called a change in the state of a gas in which its temperature remains constant.
For a constant mass of gas at a constant temperature, the product of gas pressure and volume is a constant value:
The same law can be rewritten in a different form (for two states of an ideal gas):
This law follows from the Mendeleev - Clapeyron equation:
![]()
Obviously, at a constant gas mass and at a constant temperature, the right-hand side of the equation remains constant.
The graphs of the dependence of gas parameters at constant temperature are called isotherms.
Denoting the constant by a letter, we write down the functional dependence of pressure on volume in an isothermal process:
It can be seen that the gas pressure is inversely proportional to its volume. Inverse proportionality graph, and, consequently, the graph of the isotherm in coordinates is the hyperbola(Fig. 1, a). Figures 1 b) and c) show isotherms in coordinates and, respectively.

Fig. 1. Graphs of isothermal processes in various coordinates
Gay-Lussac's law (isobaric process)
Isobaric process is called a change in the state of a gas in which its pressure remains constant.
For a constant mass of gas at constant pressure, the ratio of gas volume to temperature is a constant value:
This law also follows from the Mendeleev - Clapeyron equation:
![]()
isobars.
Consider two isobaric processes with pressures and title = "(! LANG: Rendered by QuickLaTeX.com" height="18" width="95" style="vertical-align: -4px;">. В координатах и изобары будут иметь вид прямых линий, перпендикулярных оси (рис.2 а,б).!}
Let us define the form of the graph in coordinates. Denoting the constant by a letter, we write down the functional dependence of the volume on temperature in the isobaric process:
It can be seen that at constant pressure, the volume of the gas is directly proportional to its temperature. The graph of direct proportionality, and, therefore, an isobar plot in coordinates is a straight line passing through the origin(Fig. 2, c). In reality, at sufficiently low temperatures, all gases turn into liquids, to which the gas laws are no longer applicable. Therefore, near the origin of coordinates, the isobars in Fig. 2, c) are shown with a dotted line.

Fig. 2. Graphs of isobaric processes in various coordinates
Charles's law (isochoric process)
Isochoric process is called a change in the state of a gas in which its volume remains constant.
For a constant mass of gas at a constant volume, the ratio of gas pressure to its temperature is a constant value:
For two states of gas, this law will be written in the form:
This law can also be obtained from the Mendeleev - Clapeyron equation:
![]()
The graphs of the dependence of gas parameters at constant pressure are called isochores.
Consider two isochoric processes with volumes and title = "(! LANG: Rendered by QuickLaTeX.com" height="18" width="98" style="vertical-align: -4px;">. В координатах и графиками изохор будут прямые, перпендикулярные оси (рис.3 а, б).!}
To determine the type of graph of the isochoric process in coordinates, we denote a constant in Charles's law by a letter, we get:
Thus, the functional dependence of pressure on temperature at a constant volume is a direct proportionality, the graph of such a dependence is a straight line passing through the origin of coordinates (Fig. 3, c).

Fig. 3. Graphs of isochoric processes in various coordinates
Examples of problem solving
EXAMPLE 1
| Exercise | To what temperature should a certain mass of gas with an initial temperature be cooled isobarically so that the volume of the gas decreases by one quarter? |
| Solution | The isobaric process is described by Gay-Lussac's law: According to the condition of the problem, the volume of gas due to isobaric cooling decreases by one quarter, therefore: whence the final gas temperature: Let's convert units to SI system: initial gas temperature. Let's calculate:
|
| Answer | The gas must be cooled to temperature. |
EXAMPLE 2
| Exercise | A closed vessel contains gas at a pressure of 200 kPa. What will the gas pressure be if the temperature is increased by 30%? |
| Solution | Since the container with gas is closed, the volume of gas does not change. The isochoric process is described by Charles's law: According to the condition of the problem, the gas temperature increased by 30%, so you can write: Substituting the last ratio in Charles's law, we get: Let's convert units to SI system: initial gas pressure kPa = Pa. Let's calculate: |
| Answer | The gas pressure becomes 260 kPa. |
EXAMPLE 3
| Exercise | The oxygen system that the aircraft is equipped with has |
| Solution | The isothermal process is described by the Boyle-Mariotte law: |
Boyle's Law - Mariotte
Boyle's Law - Mariott is one of the main gas laws, discovered in 1662 by Robert Boyle and independently rediscovered by Edm Mariotte in 1676. Describes the behavior of a gas in an isothermal process. The law is a consequence of the Clapeyron equation.
- 1 Formulations
- 2 Consequences
- 3 See also
- 4 Notes
- 5 Literature
The wording
The Boyle-Mariotte law is as follows:
At constant temperature and gas mass, the product of gas pressure and its volume is constant.
In mathematical form, this statement is written in the form of the formula
where is the gas pressure; is the volume of gas, and is a constant value under the agreed conditions. In general, the value is determined by the chemical nature, mass and temperature of the gas.
Obviously, if the subscript 1 denotes the quantities related to the initial state of the gas, and the subscript 2 - to the final state, then the above formula can be written in the form
.
From the above and the above formulas, the form of the dependence of the gas pressure on its volume in the isothermal process follows:
This dependence is another, equivalent to the first, expression of the content of the Boyle - Mariotte law. It means that
The pressure of a mass of gas at a constant temperature is inversely proportional to its volume.
Then the relationship between the initial and final states of the gas participating in the isothermal process can be expressed as:
It should be noted that the applicability of this and the above formula, which connects the initial and final pressures and volumes of gas with each other, is not limited to the case of isothermal processes. The formulas remain valid in those cases when the temperature changes during the process, but as a result of the process, the final temperature turns out to be equal to the initial one.
It is important to clarify that this law is valid only in cases where the gas in question can be considered ideal. In particular, the Boyle - Mariotte law is fulfilled with high accuracy in relation to rarefied gases. If the gas is strongly compressed, then significant deviations from this law are observed.
Boyle's law - Mariotte, Charles's law and Gay-Lussac's law, supplemented by Avogadro's law, are a sufficient basis for obtaining the equation of state for an ideal gas.
Consequences
Boyle's Law - Mariotte states that the pressure of a gas in an isothermal process is inversely proportional to the volume occupied by the gas. If we take into account that the density of a gas is also inversely proportional to the volume it occupies, then we come to the conclusion:
In an isothermal process, the gas pressure changes in direct proportion to its density.
It is known that compressibility, that is, the ability of a gas to change its volume under the influence of pressure, is characterized by the compressibility coefficient. In the case of an isothermal process, one speaks of the isothermal compressibility coefficient, which is determined by the formula
where the subscript T means that the partial derivative is taken at constant temperature. Substituting into this formula the expression for the relationship between pressure and volume from the Boyle - Mariotte law, we get:Thus, we come to the conclusion:
The isothermal coefficient of compressibility of an ideal gas is equal to the reciprocal of its pressure.
see also
- Gay Lussac's Law
- Charles law
- Avogadro's law
- Ideal gas
- Ideal gas equation of state
Notes (edit)
- Boyle - Mariotte's law // Physical encyclopedia / Ch. ed. A.M. Prokhorov. - M .: Soviet encyclopedia, 1988 .-- T. 1. - S. 221-222. - 704 p. - 100,000 copies
- Sivukhin D.V. General course of physics. - M .: Fizmatlit, 2005 .-- T. II. Thermodynamics and Molecular Physics. - S. 21-22. - 544 p. - ISBN 5-9221-0601-5.
- 1 2 Elementary textbook of physics / Ed. G. S. Landsberg. - Moscow: Nauka, 1985. - T. I. Mechanics. Heat. Molecular physics. - S. 430 .-- 608 p.
- 1 2 3 Kikoin A.K., Kikoin I.K., Molecular Physics. - M .: Nauka, 1976 .-- S. 35-36.
- At constant mass.
- Livshits L. D. Compressibility // Physical encyclopedia / Ch. ed. A.M. Prokhorov. - M .: Great Russian Encyclopedia, 1994 .-- T. 4. - S. 492-493. - 704 p. - 40,000 copies
ISBN 5-85270-087-8.
Literature
- Petrushevsky F.F. Boyle-Mariotte law // Brockhaus and Efron Encyclopedic Dictionary: in 86 volumes (82 volumes and 4 additional) - SPb., 1890-1907.
Boyle's Law - Marriott Information About
Boyle's Law - Mariotte
Boyle's Law - Mariotte
Boyle's Law - Mariotte You are browsing subject
Boyle's law - Mariotte what, Boyle's law - Mariotte who, Boyle's law - Mariotte description
There are excerpts from wikipedia on this article and video
Our site has a system in the function of a search engine. Above: "what were you looking for?" You can query everything in the system with the box. Welcome to our simple, stylish and fast search engine, which we have prepared to provide you with the most accurate and up-to-date information.
A search engine designed for you delivers you the most relevant and accurate information with a simple design and fast functioning system. You can find almost any information you are looking for on our website.
At the moment we only serve in English, Turkish, Russian, Ukrainian, Kazakh and Belarusian.
New languages will be added to the system very soon.
The lives of famous people give you information, images and videos on hundreds of topics such as politicians, government officials, doctors, websites, plants, technology vehicles, cars, and more.
Boyle-Mariotte law

The quantitative relationship between the volume and pressure of a gas was first established by Robert Boyle in 1662 * Boyle-Mariotte's law states that at a constant temperature, the volume of a gas is inversely proportional to its pressure.
This law applies to any fixed amount of gas. As can be seen from Fig. 3.2, its graphical representation can be different. The graph on the left shows that at low pressure, the volume of a fixed amount of gas is large.
The volume of the gas decreases as its pressure rises. Mathematically, it is written like this:
However, usually the Boyle-Mariotte law is written in the form
Such a record allows, for example, knowing the initial gas volume V1 and its pressure p, to calculate the pressure p2 in the new volume V2.
Gay Lussac's Law (Charles's Law)
In 1787 Charles showed that at constant pressure the volume of gas changes (in proportion to its temperature. This dependence is shown in graphical form in Fig. 3.3, which shows that the volume of gas is linearly related to its temperature. In mathematical form, this dependence is expressed as :
Charles's law is often written in a different form:
V1IT1 = V2T1 (2)
Charles's law was improved by J. Gay-Lussac, who in 1802 established that the volume of a gas when its temperature changes by 1 ° С changes by 1/273 of the volume that it occupied at 0 ° С.
Hence it follows that if we take an arbitrary volume of any gas at 0 ° С and at constant pressure reduce its temperature by 273 ° С, then the final volume will be equal to zero. This corresponds to a temperature of -273 ° C, or 0 K. This temperature is called absolute zero. In reality, it cannot be achieved. In fig.
3.3 shows how the extrapolation of the gas volume versus temperature plots leads to zero volume at 0 K.
Absolute zero is, strictly speaking, unattainable. However, under laboratory conditions, it is possible to reach temperatures that differ from absolute zero by only 0.001 K. At such temperatures, the random movements of molecules practically cease. This leads to amazing properties.
For example, metals cooled to temperatures close to absolute zero almost completely lose their electrical resistance and become superconducting *. An example of substances with other unusual low temperature properties is helium.
At temperatures close to absolute zero, the viscosity of helium disappears and it becomes superfluid.* In 1987, substances were discovered (ceramics sintered from oxides of lanthanide elements, barium and copper), which become superconducting at relatively high temperatures, of the order of 100 K (- 173 ° C). These "high-temperature" superconductors open up great prospects in technology. transl.
The main laboratory equipment is the desktop on which all the experimental work is carried out.
Every laboratory must have good ventilation. A fume hood is required, in which all work is carried out using foul-smelling or poisonous compounds, as well as burning organic substances in crucibles.
Highly volatile, harmful or foul-smelling substances (liquid bromine, concentrated nitric and hydrochloric acids, etc.)
), as well as flammable substances (carbon disulfide, ether, benzene, etc.).
The laboratory requires plumbing, sewerage, technical current, gas and water heating devices. It is also desirable to have a supply of compressed air, a vacuum line, a supply of hot water and steam.
If there is no special liner, water heaters of various systems are used to obtain hot water.
With these devices, heated by electricity or gas, you can quickly get a jet of hot water with a temperature of almost 100 ° C.
The laboratory must have installations for distillation (or demineralization) of water, since it is impossible to work in a laboratory without distilled or demineralized water. In cases where it is difficult or impossible to obtain distilled water, commercial distilled water is used.
Near work tables and water sinks, there must be clay jars with a capacity of 10-15 liters for draining unnecessary solutions, reagents, etc., as well as baskets for broken glass, paper and other dry waste.
In addition to desks, the laboratory should have a desk where all notebooks and notes are kept, and, if necessary, a title table. There should be tall stools or chairs near work tables.
Analytical balances and instruments requiring stationary installation (electrometric, optical, etc.) are placed in a separate room associated with the laboratory, and a special weighing room should be allocated for the analytical balance. It is desirable that the weighing scale is located with windows to the north. This is important because the balance must not be exposed to sunlight (“Scales and Weighing”).
In the laboratory, you also need to have the most necessary reference books, manuals and textbooks, since often during work there is a need for a tone or other reference.
see also
Page 3
Chemical glassware used in laboratories can be divided into a number of groups. According to the purpose, the dishes can be divided into general-purpose, special-purpose and volumetric dishes. By material - for dishes made of simple glass, special glass, quartz.
To the group. general purpose are those items that should always be in laboratories and without which most work cannot be carried out. These are: test tubes, funnels simple and separating, beakers, flat-bottomed flasks, crystallizers, conical flasks (Erlenmeyer), Bunsen flasks, refrigerators, retorts, flasks for distilled water, tees, taps.
The special-purpose group includes those items that are used for any one purpose, for example: the Kipp apparatus, the Sok-rally apparatus, the Kjeldahl apparatus, reflux condensers, Wul-fa flasks, Tishchenko flasks, pycnometers, hydrometers, Drexel flasks, potassium apparatus , carbon dioxide analyzer, round bottom flasks, special refrigerators, molecular weight instrument, melting and boiling point instruments, etc.
Volumetric instruments include: graduated cylinders and beakers, pipettes, burettes, and volumetric flasks.To begin with, we suggest watching the following video, where the main types of chemical glassware are briefly and easily considered.
see also:
General purpose cookware
Test tubes (Fig. 18) are narrow cylindrical vessels with a rounded bottom; they come in different sizes and diameters and are made of different glass. Ordinary "laboratory test tubes are made of low-melting glass, but for special work, when heating to high temperatures is required, test tubes are made of high-melting glass or quartz.
In addition to ordinary, simple tubes, graduated and centrifuge conical tubes are also used.
Special wooden, plastic or metal racks are used to store test tubes in operation (Fig. 19).
Rice. 18. Simple and graduated tubes
Rice. 20. Putting a tag of powdered substances into the test tube.
Test tubes are used mainly for analytical or microchemical work. When carrying out reactions in a test tube, reagents should not be used in too large quantities. It is absolutely unacceptable for the test tube to be filled to the brim.
The reaction is carried out with small amounts of substances; 1/4 or even 1/8 of the capacity of the test tube is enough. Sometimes a solid substance (powders, crystals, etc.) needs to be injected into the test tube.
), for this a strip of paper with a width slightly less than the diameter of the test tube is folded in half along its length and the required amount of solid is poured into the resulting scoop. The test tube is held in the left hand, tilting it horizontally, and the scoop is inserted into it almost to the bottom (Fig. 20).
Then the test tube is placed vertically, but also lightly hit on it. When all the solids have spilled out, remove the paper scoop.
To mix the poured reagents, the test tube is held by the upper end with the thumb and forefinger of the left hand and supported with the middle finger, and the bottom of the tube is struck with the index finger of the right hand. This is enough for its contents to be well mixed.
It is absolutely unacceptable to close the test tube with your finger and shake it like this; in this case, you can not only introduce something foreign into the liquid in the test tube, but sometimes damage the skin of the finger, get a burn, etc.
If the tube is more than half full of liquid, stir the contents with a glass rod.
If the tube needs to be heated, it must be clamped in the holder.
With inept and strong heating of the test tube, the liquid quickly boils and splashes out of it, so you need to heat it carefully.When bubbles begin to appear, the test tube should be set aside and, holding it not in the burner flame, but near or above it, continue heating with hot air. When heated, the open end of the test tube should be directed away from the worker and from neighbors on the table.When no strong heating is required, it is better to immerse the tube with the heated liquid in hot water. If working with small test tubes (for semi-microanalysis), then they are heated only in hot water poured into a glass beaker of the appropriate size (with a capacity not exceeding 100 ml).
Funnels are used for pouring - liquids, for filtering, etc. Chemical funnels are produced in various sizes, their top diameters are 35, 55, 70, 100, 150, 200, 250 and 300 mm.
Conventional funnels have a smooth inner wall, but funnels with a ribbed inner surface are sometimes used for faster filtration.
The filter funnels always have an angle of 60 ° and a cut-off long end.
During operation, the funnels are installed either in a special tripod or in a ring on an ordinary laboratory tripod (Fig. 21).
For filtering into a glass, it is useful to make a simple holder for the funnel (Fig. 22). For this, a strip 70-80 lsh long and 20 mm wide is cut out of sheet aluminum with a thickness of about 2 mm.
A hole with a diameter of 12-13 mm is drilled at one of the ends of the strip and the strip is bent as shown in Fig. 22, a. How to fix the funnel on the glass is shown in fig. 22, b.
When pouring liquid into a bottle or flask, do not fill the funnel to the brim.
If the funnel fits snugly against the throat of the vessel into which the liquid is poured, then the transfer becomes difficult, since an increased pressure is created inside the vessel. Therefore, the funnel needs to be lifted from time to time.
It is even better to make a gap between the funnel and the neck of the vessel by placing, for example, a piece of paper between them. In this case, care must be taken that the gasket does not get into the vessel. It is more advisable to use a wire triangle, which you can make yourself.This triangle is placed on the neck of the vessel and then the funnel is inserted.
There are special rubber or plastic attachments on the neck of the dishes, which ensure the communication of the inside of the flask with the outside atmosphere (Fig. 23).
Rice. 21. Strengthening the glassy chemical funnel
Rice. 22. Device for fixing the funnel on a glass, in a tripod.
For analytical work when filtering, it is better to use analytical funnels (Fig. 24). The peculiarity of these funnels is that they have an elongated cut end, the inner diameter of which is smaller in the upper part than in the lower part; this design speeds up filtration.
In addition, there are analytical funnels with a ribbed inner surface supporting the filter, and with a spherical expansion at the point where the funnel passes into the tube. Funnels of this design accelerate the filtration process up to three times compared to conventional funnels.
Rice. 23. Nozzles for bottle necks. Rice. 24. Analytical funnel.
Separating funnels(fig. 25) are used to separate immiscible liquids (eg water and oil). They are either cylindrical or pear-shaped and in most cases are equipped with a glass stopper.
In the upper part of the branch pipe there is a glass ground tap. The capacity of the separating funnels is different (from 50 ml to several liters), depending on the capacity, the wall thickness also changes.
The smaller the funnel capacity, the thinner its walls, and vice versa.
During operation, the separating funnels are strengthened in different ways, depending on the capacity and shape. The cylindrical funnel of small capacity can be simply attached to the foot. Large funnels are placed between two rings.
The lower part of the cylindrical funnel should rest on a ring, the diameter of which is slightly less than the diameter of the funnel, the upper ring has a slightly larger diameter.
If the funnel is swinging at the same time, a plate from the cork should be placed between the ring and the funnel.
A pear-shaped dividing funnel is fixed on a ring, its neck is clamped with a paw. Always fix the funnel first, and only then pour the liquids to be separated into it.Drip funnels (Fig. 26) differ from dividing funnels in that they are lighter, thin-walled and
Rice. 25. Separation funnels. rice. 26. Drip funnels.
In most cases with a long end. These funnels are used in many works, when a substance is added to the reaction mass in small portions or dropwise. Therefore, they usually form part of the device. Funnels are fixed in the neck of the flask on a thin section or with a cork or rubber stopper.
Before working with a separating or dropping funnel, the glass tap section must be carefully lubricated with petroleum jelly or special grease.
This makes it possible to open the tap easily and effortlessly, which is very important, since if the tap opens tightly, it can break it when opening it or damage the entire device.
The grease should be applied in a very thin layer so that when turning the valve, it does not fall into the funnel tube or inside the valve opening.
For a more uniform flow of liquid droplets from a dropping funnel and to monitor the rate of liquid supply, dropping funnels with a nozzle are used (Fig. 27). Such funnels have an expanded part immediately after the tap, which turns into a tube. The liquid flows through the cock into this expansion through a short tube and then into the tube of the funnel.
Rice. 27. Drip funnel with nozzle
Rice. 28. Beakers.
Rice. 29. Flat funnel with nozzle
GLASS WARE 1 2 3
see also
Lesson 25. Boyle-Mariotte's Law - HIMI4KA

Lessons Archive ›Basic Laws of Chemistry
Lesson 25 “ Boyle-Mariotte law"From the course" Chemistry for dummies»Consider the law relating pressure and volume of gas, as well as graphs of the dependence of pressure on volume and volume on pressure. Let me remind you that in the last lesson "Gas pressure" we examined the device and principle of operation of a mercury barometer, and also gave a definition of pressure and considered its units of measurement.
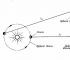
Robert Boyle(1627-1691), to whom we owe the first practically correct definition of a chemical element (we learn in Chapter 6), was also interested in the phenomena occurring in vessels with rarefied air.
Inventing vacuum pumps for pumping air out of closed vessels, he drew attention to a property familiar to everyone who has happened to pump a soccer ball chamber or carefully squeeze a balloon: the more the air is compressed in a closed vessel, the more it resists compression.
Boyle called this property “ springiness»Air and measured it with a simple device shown in fig. 3.2, a and b.
Boyle blocked some air with mercury at the closed end of the curved tube (Figure 3-2, a) and then squeezed this air, gradually adding mercury to the open end of the tube (Figure 3-2, b).
The pressure experienced by the air in the closed part of the tube is equal to the sum of the atmospheric pressure and the pressure of a column of mercury with a height h (h is the height at which the level of mercury at the open end of the tube exceeds the level of mercury at the closed end). Boyle's obtained pressure and volume measurements are shown in Table 1. 3-1.
Although Boyle did not take special measures to maintain a constant temperature of the gas, it seems that in his experiments it changed only slightly. Nevertheless, Boyle noticed that the heat from the candle flame caused significant changes in the properties of the air.
Analysis of data on the pressure and volume of air during its compression
Table 3-1, which contains Boyle's experimental data on the relationship between pressure and volume for atmospheric air, is located under the spoiler.
After the researcher receives data similar to those shown in table. 3-1, he tries to find a mathematical equation that relates the two dependent quantities that he measured.
One way to obtain such an equation is to graphically plot the dependence of various degrees of one quantity on another, in the hope of obtaining a straight line graph.
The general equation of a straight line is:
where x and y are related variables, and a and b are constant numbers. If b is zero, a straight line goes through the origin.
In fig. 3-3 show different ways of graphing data for pressure P and volume V, shown in table. 3-1.Plots of P versus 1 / K and V versus 1 / P are straight lines passing through the origin.
The plot of the dependence of the logarithm of P on the logarithm of V is also a straight line with a negative slope, the tangent of which is -1. All these three plots lead to equivalent equations:
- P = a / V (3-3a)
- V = a / P (3-3b)
- lg V = lg a - lg P (3-3v)
Each of these equations represents one of the options Boyle-Mariotte law, which is usually formulated as follows: for a given number of moles of gas, its pressure is proportional to the volume, provided that the gas temperature remains constant.
By the way, you are probably wondering why the Boyle-Mariotte law is called by a double name. This happened because this law, independently of Robert Boyle, who discovered it in 1662, was rediscovered by Edm Mariotte in 1676. So that's it.
When the relationship between two measured quantities is simple to the extent that it is in this case, it can also be established numerically.
If each value of pressure P is multiplied by the corresponding value of volume V, it is easy to make sure that all products for a given gas sample at constant temperature are approximately the same (see Table 3-1). Thus, we can write that
Equation (З-Зг) describes the hyperbolic relationship between the values of P and V (see Fig. 3-3, a). To check that the plot of the dependence of P on V constructed from the experimental data really corresponds to the hyperbola, we will construct an additional plot of the dependence of the product P V on P and make sure that it is a horizontal straight line (see Fig. 3-3, e) ...
Boyle found that for a given amount of any gas at a constant temperature, the relationship between pressure P and volume V is quite satisfactorily described by the relation
- P V = const (at constant T and n) (3-4)
Boyle-Mariotte formula
To compare volumes and pressures of the same gas sample under different conditions (but constant temperature), it is convenient to represent Boyle-Mariotte law in the following formula:
where indices 1 and 2 correspond to two different conditions.
Example 4. Plastic bags with foodstuffs delivered to the Colorado plateau (see example 3) often burst, because the air in them, when rising from sea level to an altitude of 2500 m, under conditions of reduced atmospheric pressure, expands.
If we assume that there is 100 cm3 of air inside the bag at atmospheric pressure corresponding to sea level, how much volume should this air occupy at the same temperature on the Colorado Plateau? (Assume that shrunken pouches are used to deliver products that do not restrict air expansion; missing data should be taken from Example 3.)Solution
Let's use Boyle's law in the form of equation (3-5), where index 1 will refer to conditions at sea level, and index 2 - to conditions at an altitude of 2500 m above sea level. Then Р1 = 1,000 atm, V1 = 100 cm3, Р2 = 0.750 atm, and V2 should be calculated. So,
The study of the relationship between the parameters characterizing the state of a given mass of gas, we begin with the study of gas processes occurring when one of the parameters remains unchanged. English scientist Boyle(in 1669) and a French scientist Marriott(in 1676) discovered a law that expresses the dependence of the change in pressure on the change in the volume of gas at a constant temperature. Let's carry out the following experiment.
By rotating the handle, we will change the volume of gas (air) in cylinder A (Fig. 11, a). According to the reading of the manometer, we note that the gas pressure also changes. We will change the volume of gas in the vessel (the volume is determined on the B scale) and, noticing the pressure, we will write them down in table. 1. It can be seen from it that the product of the gas volume by its pressure was almost constant: how many times "the gas volume decreased, the same time its pressure increased.

As a result of similar, more accurate, experiments, it was discovered: for a given mass of gas at a constant temperature, the gas pressure changes in inverse proportion to the change in the volume of the gas. This is the formulation of the Boyle-Mariotte law. Mathematically, it will be written for two states as follows:
![]()
The process of changing the state of a gas at a constant temperature is called isothermal. The Boyle-Mariotte formula is the equation for the isothermal state of a gas. At a constant temperature, the average speed of movement of molecules does not change. A change in the volume of gas causes a change in the number of impacts of molecules on the walls of the vessel. This is the reason for the change in gas pressure.
Let us depict this process graphically, for example, for the case V = 12 liters, p = 1 at.... We will plot the gas volume on the abscissa axis, and its pressure on the ordinate axis (Fig. 11, b). We find the points corresponding to each pair of values of V and p, and, connecting them together, we get a graph of the isothermal process. The line showing the relationship between the volume and pressure of a gas at constant temperature is called an isotherm. Pure isothermal processes do not occur. But it is not uncommon for the gas temperature to change little, for example, when the compressor is pumping air into the cylinders, when the combustible mixture is injected into the cylinder of an internal combustion engine. In such cases, the gas volume and pressure are calculated according to the Boyle-Mariotte law *.