Cytochrome p450 characteristics and biological role. P450 cytochromes
Polunina T.E.
Oksana Mikhailovna Drapkina
– We continue our program. Our lectures and discussions on gynecology are ending, we have completely entered into the regulations, so we will try not to leave them. Professor Tatyana Evgenievna Polunina opens the section of gastroenterology. Lectures “The role of the cytochrome P450 family in the pathogenesis and treatment of non-alcoholic fatty liver disease.”
Tatyana Evgenievna Polunina, professor, doctor of medical sciences:
– Cytochromes P450 (CYP 450) is the name of a large family of universal enzymes in the human body. Cytochromes P450 play an important role in the oxidation of numerous compounds, such as endogenous compounds (steroids, bile acids, fatty acids, prostaglandins, leukotrienes, biogenic amines), as well as exogenous compounds (drugs, industrial pollution products, pesticides, carcinogens and mutagens), the latter are called xenobiotics.
In this slide you can see where the cytochromes P450 are located. They are located in the hepatocyte, in the cytosol. The endoplasmic reticulum is the basis for the location. And, in particular, the lipid membrane, which contains a bilayer of phospholipids, has several connected structures on it. This is a cytochrome, which includes iron protein, nicotinamide adenine dinucleotide and oxidoreductase, which is included in the complex of metabolism of drugs and the above presented xenobiotics.
The most common representatives of this group that clinicians turn to are cytochromes P452 AC, P450 2D, P450 2E1, P450 3A4. These enzymes catalyze a wide range of metabolic reactions and one cytochrome can metabolize several drugs that have different chemical structures. The same drug has different effects in cytochrome P450 and in different organs. And, in particular, the most important cytochrome that we pay attention to is cytochrome P450 2E - the most important isoenzyme of cytochrome P450, it breaks down low-density lipoproteins.
Currently, not only phenotyping methods have been developed that are based on the substrate specificity of certain cytochrome P450 isoenzymes, but also the activity of a particular enzyme and metabolism is determined by the pharmacokinetics of the marker substrate and changes in the concentrations of the unchanged substance and its metabolite. But the determination of cytochrome P450 isoenzymes by identifying the genes for the corresponding isoenzymes is carried out using a polymerase chain reaction. This is called cytochrome P450 isoenzyme genotyping.
On this slide we see that in the hepatocyte the place where the endoplasmic reticulum, cytochromes P450, of which there are more than 50, and drugs that are broken down in a certain cytochrome are located, in some cases it combines with the cytochrome and forms a vesicle that damages the hepatocyte, causing at the same time stress and cytokines; leads to activation of the tumor necrotic factor and, in particular, is a trigger factor for the launch of caspases, which manifests itself with catalytic processes.
Non-alcoholic fatty liver disease, which was subsequently identified as a nosological entity, began to be called non-alcoholic fatty liver disease (NAFLD) since 1980, after discovering changes in the liver of non-alcoholic patients that were similar to those seen in alcohol-induced damage.
The natural history of non-alcoholic fatty liver disease includes steatosis as an initial stage, which, without progressing, can be asymptomatic, and steatohepatitis, which is accompanied by terrible vegetative manifestations, cytolysis syndrome and dyspeptic manifestations. With the development of fibrosis, a rather serious problem arises - liver cirrhosis, and subsequently portal hypertension and carcinoma develop.
I would like to draw your attention to the fact that back in 1894, Kiernan proposed a certain liver architecture, which consists of a beam structure. On the periphery of the beams, which consist of polygonal hepatocytes, there is a triad: bile duct, portal vein and artery. This slide represents a normal healthy liver and fatty infiltration of hepatocytes. Liver steatosis, which is one of the first phases of development of non-alcoholic fatty liver disease, is presented in morphological form in this diagram.
The next option for the development of the inflammatory process, which leads to fibrous tissue spreading throughout the liver, we see steatohepatitis and subsequently cirrhosis of the liver with the development of portal hypertension. Most often, this is micronodular cirrhosis of the liver, which is already quite clearly established in the stages of development of non-alcoholic fatty liver disease, it is accompanied by portal hypertension, varicose veins of the esophagus, stomach, complications that are typical for cirrhosis of the liver, and death.
With non-alcoholic steatohepatitis, the most common developments are those that are most often associated as concomitant diseases: diabetes mellitus, obesity. In patients, non-alcoholic steatohepatitis develops up to 75%, and if diabetes mellitus and obesity are combined, then 90% of patients have non-alcoholic fatty liver disease.
The liver is undoubtedly the main target organ affected by metabolic syndrome. Insulin resistance is a key feature that is the basis for intrahepatocyte lipid accumulation, fatty liver, non-alcoholic steatohepatitis and liver cirrhosis.
I would like to draw attention to the fact that metabolic syndrome includes not only impaired glucose tolerance, but also dyslipidemia, abdominal-visceral obesity, insulin resistance and hyperinsulinemia, arterial hypertension, early atherosclerosis, impaired hemostasis, hyperuricemia, hyperandrogenism. I would like to say that non-alcoholic fatty liver disease, steatosis, is part of the metabolic syndrome and is currently a quintet that used to be called the “deadly quartet”.
The risk factors presented on this slide sometimes vary from country to country, with US positions and European positions differing slightly. But, nevertheless, waist circumference, levels of triglycerides, lipoproteins, blood pressure, in particular 130/85, glucose levels are indicators that must be monitored in a patient with metabolic syndrome.
Diseases associated with lipid metabolism are: non-alcoholic fatty liver disease, type 2 diabetes mellitus, coronary liver disease, hypertension.
In the pathogenesis scheme, insulin resistance of adipose tissue is of particular importance. An increase in lipogenesis, that is, an increase in the level of fatty acids, an increase in the synthesis of triglycerides and lipotoxicity lead to the development of insulin resistance, and this leads to metabolic dysfunction, stress of the endoplasmic reticulum, in which the metabolism of fatty acids and in particular lipoproteins also occurs, and to the activation of inflammation . These are Kupffer cells and stellate cells, which further lead not only to the fact that the level of very low density lipids increases, but undoubtedly this leads to the development of steatohepatitis with fibrosis, and we get the activity of a process that moves towards cirrhosis of the liver.
At the hepatocyte level, fatty acids undergo esterification into triglycerides and are exported as low-density lipoproteins, a situation in the normal hepatocyte that is associated with oxidation in mitochondria, peroxisomes and microsomes.
Undoubtedly, in the mechanism of insulin resistance, which is presented here, a key role belongs to the tumor necrotic factor, free radicals, leptin, fatty acids and increased lipolysis, which leads to the absorption of fatty acids, to a violation of β-oxidation of fatty acids in mitochondria and also to the accumulation of fatty acids in hepatocyte.
Induction of cytochromes P450 4A11 and P450 2E1 leads to lipid peroxidation, which undoubtedly leads to the activation of factors associated with the accumulation of triglycerides. Hyperinsulinemia is a key factor that leads to insulin resistance. It also leads to an increase in glycolysis, fatty acid synthesis and triglyceride accumulation in hepatocytes.
The next slide shows the mechanism of interaction between microsomal oxidation and mitochondrial β-oxidation. Note that mitochondrial Ω-oxidation and mitochondrial β-oxidation lead to the triggering of so-called peroxisomal β-oxidation receptors and in particular peroxisome proliferator-activated receptors. This leads to the expression of the accumulation of a certain protein and, accordingly, acetyl-coenzyme A, which accumulates and triggers a mechanism that leads to an overload of dicarboxylic fatty acids.
In the next slide you see that steatohepatitis and fibrosis are formed against the background of mitochondrial reactive oxygen species. The key to triggering fibrosis is undoubtedly the accumulation of malondialdehyde, which leads to the formation of inflammatory infiltrates, fibrosis and activation of stellate cells. Stellate cells trigger the induction of cytokines such as tumor necrotic factor and transforming growth factors. Depletion of the antioxidant system leads to the launch of Fas-legand, a mitochondrial reactive oxygen species, necrosis of the hepatocyte occurs, and fibrous tissue subsequently develops, which is the basis for the development of cirrhosis.
This slide shows a diagram; you see excess lipids that accumulate in the hepatocyte. Mitochondrial dysfunction and dysfunction of cytochrome P450 leads to activation of lipid peroxidation, the launch of Kupffer cells, inflammatory cytokines, activation of stellate cells and apoptosis, which subsequently leads to the development of hepatocyte necrosis.
Metabolic syndrome is very important because non-alcoholic fatty liver disease is part of the metabolic syndrome. And not only on the hepatocyte, in which there is an increase in the level of low-density and very low-density lipoproteins, triglycerides (this is very important), but also on the endothelial cell. Endothelial dysfunction occurs and a moment is also triggered that is associated with lipid peroxidation, the accumulation of substances that affect atherosclerosis, sudden death, and heart attacks.
Undoubtedly, the increase in free fatty acid levels is associated with adipocytes. And a decrease in esterified cholesterol in particular also leads to various stresses of the nuclear receptor. And the so-called activated peroxisome proliferator receptor is especially important at present; it is to it that all the attention of scientists who work with obesity, diabetes, and non-alcoholic fatty liver disease is directed.
A monocyte (macrophage), in some cases, by increasing the level of inflammatory responders (tumor-necrotic factor, interleukins-6, membrane toll-like receptors, free fatty acids) also triggers events that are associated specifically with the pathological effects of fatty acids.
The criteria for assessing insulin resistance have been known to everyone since 1985. It is determined by the HOMA index - Homeostasis Model Assessment, and the more modern QUICKI index - Quantitave Insulin Sensitivity. Insulin concentration, serum glucose, and norms are presented here.
We would like to point out that not all patients with non-alcoholic fatty liver disease need a liver biopsy. We currently have points that enable us to determine the level of fatty infiltration of the liver. And in particular this is a fibrotest.
In the algorithm for diagnosing non-alcoholic fatty liver disease, we pay attention not only to specific signs, but also to the activity of the enzymes alanine and aspartic transaminase, gamma-glutamyl transpeptidase, alkaline phosphatase, and we pay attention to alcohol intake, which was discussed by previous colleagues. And I would like to draw attention, of course, to risk factors: metabolic syndrome, insulin resistance, diabetes. Therapy is prescribed to correct this situation, and, if necessary, a liver biopsy. Undoubtedly, absolute indications for biopsy are required. And if the body mass index exceeds 35 and 40, then measures related to surgical treatment are already being carried out.
I would like to draw your attention to a number of medications (non-steroidal - anti-inflammatory glucocorticosis, and steroid drugs, tetracycline antibiotics), a number of nutritional factors (fasting, rapid weight loss, surgical interventions, metabolic genetic factors, in particular, hereditary hemochromatosis, various poisons) and other concomitant diseases. This is very important for differential diagnosis.
At the stage of steatosis, treatment of obesity, insulin resistance, and dyslipidemia is important. In the stage of steatohepatitis, the most important point is the elimination of oxidative stress, inflammation and fibrosis.
Excessive induction of cytochrome P450 2E has detrimental effects on hepatocytes due to the release of free radicals. Essential phospholipids act not only as antioxidants, but also serve as a very important factor for reducing the activity of cytochrome 2E1, as shown in the works of M. Aleynik. The results of some studies suggest that the introduction of essential phospholipids can reduce the induction of cytochrome P450 2E (work by Vladimir Trofimovich Ivashkin, who was presented with Marina Viktorovna Mayevskaya in Russian sources in 2004).
Stellate cells take part in the formation of the final stage of non-alcoholic fatty liver disease. And in laboratory experiments, it has been demonstrated that complete prevention of stellate cell activation using CYP2E1 inhibitors prevents the development of cirrhosis.
I would like to draw your attention to the fact that not only the Russian author M. Aleynik, but also the Japanese author Akiyama in the journal “Hepatology” in 2009, based on a model of alcoholic liver damage, also pays attention to cytochrome P450 2E, acetyl-CoA oxidase and nicotinamide adenine dinucleotide oxidases, that essential phospholipids exhibit anti-inflammatory, anti-apoptotic and anti-fibrotic activity in this pathology.
This is a theoretical version of the assumption of the use of cytochrome P450 inhibitors, and in particular the drug “Essentiale”, which is the reference, and is the most important point for the inhibition of cytochromes P450 2E and, accordingly, P450 4A11. This prevents lipid oxidation, glycolysis and reduces fatty acid synthesis.
The following drugs are used in the treatment of non-alcoholic fatty liver disease: insulin sensitizers, antioxidants, hepatoprotectors, antimicrobials.
But I would like to draw attention to membrane phospholipids. They are the main lipid components of cell membranes. Damage to phospholipid membranes leads to cytolysis syndrome, and excess reactive oxygen species leads to damage to phospholipid membranes based on microsomal γ-oxidation and peroxymal β-oxidation. Accordingly, damage to phospholipid membranes results in cell death, which leads to the initiation of fibrosis and activation of stellate cells.
Damage to the liver structure is damage to the membranes. In the version of essential phospholipids, it is a material that restores cell membranes instead of lipids. Restoring the liver structure makes it possible to restore liver function.
Our patients suffer not only from alcoholic fatty liver disease, alcoholic hepatitis, but also from other liver diseases, this is an indisputable fact. I would like to draw your attention to the fact that according to E. Kunz (2008 monograph), essential phospholipids have an antifibrotic effect, an effect that stabilizes bile and the hepatocyte membrane.
This is a publication that was released in 2008 based on pharmacological and clinical data. Therapy with essential phospholipids seems to be the preferred choice for significantly reducing the manifestations and eliminating fatty liver disease of various etiologies, which has developed due to alcohol consumption, obesity, and even if the cause cannot be discerned.
I would like to point out that there are several studies on Essential. These studies are well known to everyone. But I would like to say that even with diabetes mellitus, Essentiale makes it possible to normalize the level of glucose, glycated hemoglobin, and serum cholesterol in patients with non-alcoholic liver disease.
In conclusion, I would like to say that liver damage characterized by fat accumulation in the absence of alcohol abuse is known as non-alcoholic fatty liver disease. Risk factors are obesity and type 2 diabetes. In the pathogenesis of non-alcoholic fatty liver disease, particular importance is given to the excessive activity of cytochromes P450 2E1. Clinical variants of the course of the disease: pain in the right hypochondrium, asthenovegetative and dyspeptic disorders, hepatomegaly. And our diagnostic algorithm is based on the consistent exclusion of alcoholic and iatrogenic, as well as viral liver damage.
Microsomal oxidation is a sequence of reactions involving oxygenases And NADPH, leading to the introduction of an oxygen atom into the composition of a non-polar molecule and the appearance of hydrophilicity in it and increases its reactivity.
Reactions microsomal oxidation carried out by several enzymes located on the membranes of the endoplasmic reticulum (in the case in vitro they are called microsomal membranes). Enzymes organize short chains that end with cytochrome P 450.
Microsomal oxidation reactions include to phase 1 reactions and are intended to impart polar properties to a hydrophobic molecule and/or to increase its hydrophilicity, enhancing the reactivity of molecules to participate in phase 2 reactions. In oxidation reactions, the formation or release of hydroxyl, carboxyl, thiol and amino groups occurs, which are hydrophilic.
Microsomal oxidation enzymes are located in the smooth endoplasmic reticulum and are mixed function oxidases(monooxygenases).
Cytochrome P450
The main protein of microsomal oxidation is hemoprotein - cytochrome P 450. In nature, there are up to 150 isoforms of this protein, which oxidize about 3000 different substrates. The ratio of different cytochrome P450 isoforms varies due to genetic characteristics. It is believed that some isoforms are involved in the biotransformation of xenobiotics, while others metabolize endogenous compounds (steroid hormones, prostaglandins, fatty acids, etc.).
Cytochrome P450 interacts with molecular oxygen and includes one oxygen atom in the substrate molecule, contributing to the appearance (increasing) of its hydrophilicity, and the other - in the water molecule. Its main reactions are:
- oxidative dealkylation, accompanied by the oxidation of the alkyl group (at the N, O or S atoms) to the aldehyde and its elimination,
- oxidation (hydroxylation) of non-polar compounds with aliphatic or aromatic rings,
- oxidation of alcohols to the corresponding aldehydes.
The work of cytochrome P 450 is ensured by two enzymes:
- NADH-cytochrome b 5 oxidoreductase, contains FAD,
- NADPH-cytochrome P 450 oxidoreductase, contains FMN And FAD.

Scheme of the relative positions of microsomal oxidation enzymes and their functions
Both oxidoreductases receive electrons from the corresponding reduced equivalents and transfer them to cytochrome P 450. This protein, having previously attached a molecule of the reduced substrate, binds to an oxygen molecule. Having received another electron, cytochrome P 450 incorporates the first oxygen atom into the hydrophobic substrate (substrate oxidation). At the same time, the reduction of the second oxygen atom to water occurs.

Sequence of reactions of hydroxylation of substrates with the participation of cytochrome P450
An essential feature of microsomal oxidation is the ability to induce or inhibit, i.e. to a change in process power.
Inducers are substances that activate the synthesis of cytochrome P 450 and the transcription of the corresponding mRNA. They are
1. Wide spectrum actions that have the ability to stimulate the synthesis of cytochrome P 450, NADPH-cytochrome P 450 oxidoreductase and glucuronyl transferase. The classic representatives are barbituric acid derivatives - barbiturates, This group also includes diazepam, carbamazepine, rifampicin and etc.
2. Narrow spectrum and actions, i.e. stimulate one of the forms of cytochrome P 450 - aromatic polycyclic hydrocarbons ( methylcholanthrene, spironolactone), ethanol.
For example, ethanol stimulates the synthesis of the P 450 2E1 isoform (alcohol oxidase), which is involved in the metabolism of ethanol, nitrosamines, paracetamol, etc.
Glucocorticoids induce the P 450 3A isoform.
Inhibitors of microsomal oxidation bind to the protein part of cytochrome or heme iron. They are divided into:
1. Reversible
- directactions- carbon monoxide ( CO), antioxidants,
- indirectactions, i.e. influence through intermediate products of their metabolism, which form complexes with cytochrome P 450 - erythromycin.
2. Irreversible inhibitors – allopurinol, aminazine, progesterone, oral contraceptives, teturam, fluorouracil,
Evaluation of phase 1 reactions
Microsomal oxidation can be assessed in the following ways:
- determination of microsomal enzyme activity after biopsy,
- on the pharmacokinetics of drugs,
- using metabolic markers ( antipyrine test).
Antipyrine test
The subject takes it in the morning on an empty stomach amidopyrine at the rate of 6 mg/kg body weight. 4 portions of urine are collected at intervals from 1 to 6 hours, 6-12, 12-24 and 45-48 hours, respectively. The volume of urine is measured. No later than 24 hours later, the urine is centrifuged or filtered. Next, the concentration of 4-aminoantipyrine and its metabolite N-acetyl-4-aminoantipyrine in urine is examined.
Cytochromes P450
The cytochrome P-450 (CYP-450) superfamily is responsible for microsomal oxidation and is a group of enzymes with many isoforms (more than 1000), which not only metabolize drugs, but also participate in the synthesis of steroid hormones, cholesterol and other substances.
The largest amount of cytochromes is found in hepatocytes, as well as in organs such as the intestines, kidneys, lungs, brain, heart. Based on the homology of nucleotide and amino acid sequences, cytochrome isoenzymes are divided into families, which, in turn, are divided into subfamilies. Representatives of different families differ in substrate specificity and activity regulators (inducers and inhibitors). Although individual members of families may have "cross" specificities and "cross" inducers and inhibitors. Thus, it has been shown that the antiviral drug ritonavir is metabolized by seven enzymes (CYP1A1, CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4), and cimetidine inhibits four enzymes (CYP1A2, CYP2C9, CYP2D6, CYP3A4). The most important cytochromes for the biotransformation of drugs are CYP1A1, CYP2A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4, CYP3A5. The relative contribution of various cytochromes and other phase I detoxification enzymes in drug metabolism is presented in Figure 7.2.2.
Each cytochrome P-450 isoenzyme is encoded by its own gene, which are localized on different chromosomes. Some of these genes have pseudogenes (non-expressed copies) located close to them, which significantly complicate genetic testing.
Due to polymorphism of metabolic genes, the activity of the corresponding enzymes can vary significantly between individuals. Depending on these interindividual characteristics, three groups of individuals are distinguished, differing in the activity of one or another metabolic enzyme. These are the so-called “extensive” metabolizers - individuals with a normal rate of metabolism of drugs (the bulk of the population), “slow” metabolizers (individuals with a reduced rate of metabolism of certain drugs) and “fast” (“overactive”) metabolizers - individuals with an increased rate biotransformation of some drugs. The proportion of “slow” and “fast” metabolizers for individual metabolic enzymes reveals significant interpopulation differences. At the same time, there is not always a complete correlation between genotype and phenotype in the rate of drug metabolism, which indicates the need to use biochemical control when genotyping metabolic enzymes.
Let us consider the functional features of polymorphism of the main genes of the CYP-450 cytochrome superfamilies involved in drug metabolism. Detailed information about the properties of metabolic enzymes, their substrate characteristics and genetic polymorphism can be found in a series of domestic monographs and textbooks on clinical pharmacogenetics.
The P-450 CYP1 family metabolizes a relatively small proportion of xenobiotics, the most important of which are polycyclic aromatic hydrocarbons (PAHs), the main components of tobacco smoke.
A particularly important role in this belongs to the CYP1A1 and CYP1A2 genes, localized on chromosome 15. The expression of both genes is regulated by a complex formed by the Ah receptor with the inducing PAH molecule, which penetrates the nucleus and specifically stimulates the expression of these genes.
CYP1A1 encodes a protein with arylhydrocarbonate hydroxylase activity, which controls the initial metabolism of PAHs leading to the formation of carcinogens (for example, benzopyrene, which is formed during smoking). Gene polymorphism of CYP1A1 is caused by three point mutations: C4887A and A4889G in exon 7 and T6235C in the 3’-flanking region. The G4889(Val)+C6235 substitution is characterized by the appearance of the “fast” allele *2B. It has 3 times higher activity compared to the wild type allele. *2B occurs in almost 7% of Caucasians and is considered a risk factor for lung cancer. It has been shown that in the presence of the *2B allele in smokers, the risk of developing lung cancer compared to non-smokers increases by more than seven times. The risk becomes even greater if, in addition to the *2B allele of the CYP1A1 gene, the smoking individual also has a “deficient” allele of the GSTM1 gene. Alleles *2A (C6235) and *4 (A4887(Asp) occur in the population with a frequency of only 1-3%. Moreover, the *2A allele is associated with a hereditary predisposition to leukemia and resistance to drug therapy for this disease.
The CYP1A2 gene product metabolizes only PAHs, but also compounds such as caffeine, theophylline, etc. It has been shown that the presence of the *1A allele of the CYP1A2 gene inhibits the metabolism of drugs such as caffeine, deazepam, verapamil, methadone, theophylline, estradiol.
The P-450 CYP2 family is represented by a group of functionally most significant enzymes that metabolize a huge number of different drugs. Their activity shows a pronounced dependence on genetic polymorphism.
The CYP2A subfamily is the most important isoenzyme of this subfamily. It is involved in the conversion of nicotine to cotinine, in the hydroxylation of coumarin and cyclophosamide, and contributes to the metabolism of ritonavir, paracetamol and valproic acid. CYP2A6 is involved in the bioactivation of components of tobacco smoke - nitrosamines, which cause lung cancer. The CYP1A6 gene is localized on chromosome 19 at locus 19q13.2. The gene is mainly expressed in the liver. It has been shown that the *4 allele of the CYP1A6 gene is protective, i.e., it is associated with a lower risk of lung cancer. The presence of the *2 and *3 alleles is associated with reduced coumarin metabolism, which is important when dosing this drug due to possible hepatotoxicity.
CYP2B subfamily. All enzymes in this subfamily are induced by phenobarbital. The most significant enzyme is CYP2B6, which metabolizes many cytotoxic drugs (cyclophosphamide), antivirals (efavirenz and nevirapine), antidepressants (bupropion), anesthetics (propofol) and synthetic opioids (methadone), and is also involved in the metabolism of endogenous steroids. The CYP2B6 gene is localized in the same locus as the CYP2A6 gene and is expressed predominantly in the liver. The presence of slow alleles of the CYP2B6 gene (*2, *4, *5, *6) reduces the rate of metabolism of antiviral drugs, which leads to decreased clearance and increases the risk of complications from the central nervous system.
The CYP2C subfamily plays a key role in the metabolism of many drugs. A common property of these isoenzymes is the presence of 4-hydrolase activity against the anticonvulsant drug mephenytoin.
Particularly important for clinical pharmacogenetics is testing of polymorphism of the CYP2C9 gene located in the 10q24 locus. The gene is expressed predominantly in the liver and is the main metabolizer of angiotensin receptor inhibitors (losartan and irbersartan). Its substrates also include anticoagulants (warfarin), glucose-lowering drugs (glipizide), anticonvulsants (phenytoin, diazepam), antidepressants (amitriptyline, clomipramine, imipramine), proton pump inhibitors (omeprazole), non-steroidal anti-inflammatory drugs (diclofenac, ibuprofen, piroxicam) , tolbutamine. As mentioned, CYP2C9 gene polymorphism analysis was the first officially approved genetic test (see above). The number of individuals with reduced activity of this enzyme in the domestic population is up to 20%. At the same time, in order to avoid undesirable side effects, the therapeutic dose of the above drugs in carriers of the *2 and *3 alleles of the CYP2C9 gene must be reduced by 2-4 times.
The CYP2C19 gene is localized in the 10q24.1-q24.3 locus and is expressed in the liver. Its protein product is the main enzyme in the metabolism of proton pump inhibitors (omeprazole) and anticonvulsants (proguanil, valproic acid, diazepam, barbiturates). The frequency of its “slow” allele (*2) in the European population ranges from 5 to 200%.
CYP2D subfamily. Cytochrome CYP2D6 metabolizes about 20% of all known drugs. The CYP2D6 gene is localized on chromosome 22 at locus 22q13.1. The main site of its expression is the liver. Currently, more than 36 alleles have been identified in the CYP2D6 gene, some of them are characterized by the absence of a protein product, while others lead to the appearance of an enzyme with altered properties. Substrates of the CYP2D6 enzyme are drugs widely used in clinical practice, such as beta-blockers, antidepressants, antipsychotropic substances, antiarrhythmics, antipsychotics, antihypertensive drugs, monooxide reductase inhibitors, morphine derivatives, neurotransmitters (dopamines), analgesics, opiates. Taking into account that about 6-10% of Caucasians are slow metabolizers of this enzyme, there is an obvious need for genetic testing of CYP2D6 in order to adjust the doses of these drugs. In addition, “functionally weakened” alleles of this gene are associated with a hereditary predisposition to such serious diseases as lung cancer, intestinal cancer, etc.
CYP2E subfamily. Cytochrome CYP2E1 is an ethanolin-inducible enzyme. Its substrates are carbon tetrachloride, dimethylnitrosamine. There is evidence that CYP2E1, along with CYP1A2, is involved in the conversion of paracetamol to N-acetylbenzoquinoneimine, which has a powerful hepatotoxic effect. In addition, it is the most important isoenzyme of the group of cytochromes that oxidize low-density lipoprotein cholesterol, which, in turn, leads to the formation of atherosclerotic plaques. The CYP2E1 gene is localized at the 10q24.3-qter locus and is expressed in the liver of adult humans. Taq1 polymorphism in the CYP2E1 gene leads to a decrease in the activity of this enzyme. M/M homozygotes for the weakened allele of the CYP2E1 gene show increased sensitivity to the above drugs due to their delayed detoxification.
Cytochrome P-450 CYP3 family
The CYP3A subfamily is the most numerous. It accounts for about 30% of all cytochrome P-450 isoenzymes in the liver and 70% of all isoenzymes in the wall of the gastrointestinal tract. The most significant enzymes are CYP3A4 and CYP3A5, the genes of which are localized in the 7q22.1 locus. The CYP3A4 gene is predominantly expressed in the liver, and CYP3A5 in the gastrointestinal tract.
The CYP3A4 enzyme metabolizes over 60% of all drugs and plays a major role in the metabolism of testosterone and estrogens. Allelic variants of the CYP3A4 gene are very numerous, but data on their effect on the pharmacokinetics of the corresponding drugs are contradictory.
The CYP3A5 enzyme metabolizes some of the drugs with which CYP3A4 interacts. It has been shown that the presence of the *3 allele of the CYP3A5 gene leads to a decrease in the clearance of drugs such as alprazalam, midazolam, and saquinavir.
Paraoxonase is an enzyme responsible for the synthesis of paraoxonase, a blood plasma protein. In addition, the enzyme inactivates organophosphorus compounds, organophosphates, carbamates, and acetic acid esters. Some of these substances are chemical warfare agents - sarin, soman, tabun. Of the three known isoforms, the enzyme PON1 is the most important. Its gene is localized at locus 7q21.3. The most significant and studied polymorphism is the replacement of glutamine with arginine at position 192 (L/M polymorphism). The M allele has been shown to be associated with reduced metabolism of organophosphorus compounds.
The M allele and the M/M genotype increase the risk of developing Parkinson's disease, especially in combination with the GSTP1 gene 5 allele, and are associated with the formation of atherosclerotic plaques.
Alcohol and aldehyde dehydrogenases
Alcohol dehydrogenase is a key enzyme in the catabolism of ethanol and other alcohols, oxidizing alcohols to aldehydes. In adults, the ADH1B gene is expressed in the liver. There is a certain dynamics of its expression level depending on age. The ADH1B (ADH2) gene is localized at the 4q22 locus. The most studied polymorphism is G141A. It has been shown that allele A is associated with increased enzyme activity, which leads to excessive accumulation of intermediate metabolic products - aldehydes, which have a pronounced toxic effect. Individuals with the A allele of the ADH1B gene have increased sensitivity to ethanol and are less susceptible to alcoholism.
There are also two aldehyde dehydrogenases present in liver cells: ALDH1 (cytosolic) and ALDH2 (mitochondrial). The ALDH2 gene is localized in the 12q24.2 locus, its product plays a key role in the conversion of toxic aldehydes into the corresponding carboxylic acids, which are easily removed from the body. ALDH2 plays an important role in alcohol catabolism. It is known that among representatives of the yellow race, alcohol intoxication is caused by the absence of ALDH2 in almost 50% of the population. Polymorphism in the ALDH2 gene results in the replacement of Glu at position 487 of the protein (ALDH2*1 allele) with Lys (ALDH2*2 allele). The ALDH2*2 allele encodes an enzyme with reduced activity. In heterozygotes, enzyme activity is reduced by 10 times. The ALDH2 enzyme is involved in the pathogenesis of various cancers associated with excessive alcohol consumption - hepatocellular carcinoma, cancer of the esophagus, pharynx and oral cavity.
Intensive alcohol intake in individuals with unfavorable allelic variants of the ADH1B and ALDH2 genes can lead to the rapid development of liver complications: alcohol disease and liver cirrhosis.
Cytochrome P450 family 2 subfamily C polypeptide 9 (CYP2C9). Detection of mutation A1075C (Ile359Leu)
Gene name -CYP2C9
Localization of the gene on the chromosome– 10q23.33
- *1/*1
- *1/*3
- *3/*3
Occurrence in the population
Allele CYP2C9*3 occurs in Europeans with a frequency of 6%.
Association of marker with drug metabolism
It is being studied to determine the physiological effectiveness of the use of drugs: oral anticoagulants from the coumarin class (warfarin), sulfonylurea derivatives, non-narcotic analgesics (tenoxicam, flurbiprofen, lornoxicam, piroxicam), losartan and irbesartan (angiotensin II receptor blockers).
General information about the study
The drug most commonly used to prevent and treat thromboembolic complications is warfarin (Coumadin). It is prescribed for long-term use in a series of cases associated with increased blood clotting, as well as in the postoperative period in order to prevent the formation of blood clots due to surgery. It is often practiced to prescribe the drug to people who have suffered strokes or myocardial infarction.
To achieve the effect of drugs, they need to be bioactivated in the body (transformed into an active form) in liver cells (hepatocytes) by the cytochrome P450 (CYP) enzyme system. The genes encoding these enzymes are polymorphic, and alleles encoding the formation of enzymes with reduced or absent function are common.
The activity of cytochromes, in addition to the structural features of the genes encoding them, is influenced by factors such as age, body weight, lifestyle, bad habits, diet, concomitant diseases, and medications. These factors are responsible for the formation of individual characteristics of the work of P450 enzymes and determine the nature of the metabolism of most drugs. The main enzyme for the biotransformation of indirect anticoagulants is the cytochrome P450 isoenzyme CYP2C9.
Gene CYP2C9 localized on chromosome 10 in region 10q23.33. There are gene variants (alleles) CYP2C9, encoding the formation of an enzyme with reduced or absent function. The gene variant carrying a point substitution of adenine for cytosine at position 1075 (A1075C) leads to a decrease in the metabolic activity of the enzyme and is designated CYP2C9*3. A single nucleotide substitution results in a substitution of the amino acid isoleucine for leucine (Ile359Leu) in the CYP2C9 enzyme. Thus, an enzyme with an altered function is synthesized, the activity of which is less than 5% of the activity of the enzyme *1. The major (unchanged) variant of the gene is designated as CYP2C9*1.
The most common genotype causes normal warfarin metabolism and is designated CYP2C9 *1/*1.
Genetic marker CYP2C9*3(genotypes *3/*3 and *3/*1) is associated with a change in the functional activity of the cytochrome P450 enzyme, which reduces the rate of elimination of warfarin from the body. The presence of the *3 allele in a patient leads to a significant decrease in the activity of the cytochrome isoenzyme, which increases the anticoagulation effect of the drugs up to 7 times and can cause the development of complications such as extensive internal bleeding and episodes of excessive hypocoagulation.
Cytochromes P450. Structure and function
Among phase 1 enzymes, the leading place is occupied by the cytochrome P450 (P450 or CYP) system in terms of catalytic activity towards a huge number of xenobiotics. The highest concentration of cytochrome P450 is found in the endoplasmic reticulum of hepatocytes (microsomes). Hepatic microsomal cytochromes P450 play a critical role in determining the intensity and time of action of foreign compounds and a key role in the detoxification of xenobiotics, as well as in their activation to toxic and/or carcinogenic metabolites. Cytochrome P450-dependent monooxygenases are a multienzyme electron transport system. All cytochromes P450 are heme-containing proteins. Heme iron is usually in an oxidized state (Fe3+). By being reduced to the Fe2+ state, cytochrome P450 is able to bind ligands such as oxygen or carbon monoxide. The complex of reduced cytochrome P450 with CO has an absorption maximum at 450 nm, which was the basis for
names of these enzymes. The main reaction catalyzed by cytochromes P450 is a monooxygenase reaction, in which one oxygen atom interacts with the substrate (RH) and the other is reduced to H2O. NADPH participates as a reducing agent in the reaction:
RH (substrate) + O2 + NADPH + H+ --> ROH (product) + H2O + NADP+
The mechanism by which cytochrome receives an electron from NADPH depends on the intracellular localization of cytochrome P450. In the ER, where most of the hemoproteins involved in the biotransformation of xenobiotics are located, the electron is transferred through a flavoprotein called NADPH-P450 reductase. One reductase molecule can deliver electrons to several different P450 molecules. In mitochondria, where P450 itochromes involved in the biosynthesis of steroid hormones and vitamin D metabolism are located, the electron is transferred using 2 proteins: ferrodoxin or ferrodoxin reductase.
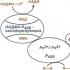
In Fig. Figure 1 shows the catalytic cycle of cytochrome P450. The 1st part of the cycle involves the activation of oxygen, the 2nd – the oxidation of the substrate. The mechanism of action of the microsomal monooxygenase system was first described by Estabrook et al., and has now been confirmed by many researchers. This scheme is as follows: the first stage consists of the interaction of the substrate with the oxidized form of P450. When P450 binds to substrates
There is a transition of heme iron from a low-spin to a high-spin state. The second stage consists of the reduction of the resulting enzyme-substrate complex with the first electron, which comes from the NADPH-specific transfer chain from NADPH through
flavoprotein I (NADPH-cytochrome P450 reductase). The third stage consists of the formation of a ternary complex: reduced cytochrome P450-substrate-oxygen. Fourth stage
represents the reduction of a ternary complex by a second electron, which, as
believed to come from the NADH-specific electron transport chain, consisting of NADH-
cytochrome b5 reductase or flavoprotein II and cytochrome b5. The fifth stage consists of several processes, including intramolecular transformations of the reduced ternary complex and its decomposition with the formation of a hydroxylated product and water. At this stage, cytochrome P450 transforms into its original oxidized form.
 Cytochromes P450 catalyze the following types of reactions: hydroxylation of an aliphatic or aromatic carbon atom; epoxidation of double bond;
Cytochromes P450 catalyze the following types of reactions: hydroxylation of an aliphatic or aromatic carbon atom; epoxidation of double bond;
oxidation of atom (S, N, I) or N-hydroxylation; transfer of oxidized group;
destruction of etheric communication; dehydrogenation. Some reactions catalyzed
cytochrome P450 are shown in Fig. 2 and 3. Several classes of reagents are good
The last carbon in the chain is hydroxylated, so-called omega-hydroxylation. So
internal hydroxylation occurs in several positions (positions -1,- 2).
 This results in many different product variations even with a simple alkane such as hexane. Note that cyclic hydrocarbons also undergo hydroxylation. In the hydroxylation reaction, a hemiacetal is first formed, which is then converted into an alcohol and an aldehyde. When alkenes are oxidized by cytochrome P450, diatomic oxides are formed. They vary in their stability and can be highly reactive. For example, vinyl chloride is metabolically converted into an oxide, which then turns into chloroacetaldehyde, a mutagen that acts directly on DNA. These studies led to a ban on the use of vinyl chloride in nebulizers. The vinyl group of sterol (vinylbenzene) is known for its carcinogenic properties, but the human body is able to neutralize it by converting the oxide into a diol using the enzyme epoxyhydrolase. But epoxyhydrolase does not always help. For example, cytochrome P450 synthesizes Aflotoxin B1 epoxide in vivo. This compound is a highly reactive electrophile, is unstable and quickly forms an adduct with DNA. In addition, the diol formed from the epoxide is also unstable and highly reactive. Oxidation of aromatic compounds with cytochrome P450 also produces epoxides, but they quickly turn into phenol. As a result of hydroxylation of benzene, the resulting phenol can be hydroxylated again, turning into catechol or hydroquinone. Note that catechol and hydroquinone can react with oxygen, inhibiting similar reactions with quinones and superoxides, which are toxins. Such a well-known compound as 2,3,7,8-tetrachlorodibenzenedioxin (TCDD) is not susceptible to hydroxylation and is stable (half-life in the human body is a year or more).
This results in many different product variations even with a simple alkane such as hexane. Note that cyclic hydrocarbons also undergo hydroxylation. In the hydroxylation reaction, a hemiacetal is first formed, which is then converted into an alcohol and an aldehyde. When alkenes are oxidized by cytochrome P450, diatomic oxides are formed. They vary in their stability and can be highly reactive. For example, vinyl chloride is metabolically converted into an oxide, which then turns into chloroacetaldehyde, a mutagen that acts directly on DNA. These studies led to a ban on the use of vinyl chloride in nebulizers. The vinyl group of sterol (vinylbenzene) is known for its carcinogenic properties, but the human body is able to neutralize it by converting the oxide into a diol using the enzyme epoxyhydrolase. But epoxyhydrolase does not always help. For example, cytochrome P450 synthesizes Aflotoxin B1 epoxide in vivo. This compound is a highly reactive electrophile, is unstable and quickly forms an adduct with DNA. In addition, the diol formed from the epoxide is also unstable and highly reactive. Oxidation of aromatic compounds with cytochrome P450 also produces epoxides, but they quickly turn into phenol. As a result of hydroxylation of benzene, the resulting phenol can be hydroxylated again, turning into catechol or hydroquinone. Note that catechol and hydroquinone can react with oxygen, inhibiting similar reactions with quinones and superoxides, which are toxins. Such a well-known compound as 2,3,7,8-tetrachlorodibenzenedioxin (TCDD) is not susceptible to hydroxylation and is stable (half-life in the human body is a year or more).