Practical work in microbiology. Workshop on Microbiology
Federal Agency for Education
State educational institution
Irkutsk State University»
SMALL PRACTICE IN MICROBIOLOGY
Study guide
Irkutsk 2009
UDC 579 (076.5)
Published by the decision of the Editorial and Publishing Council of Irkutsk State University
Zhilkin workshop on microbiology: Textbook.-method. manual for stud. higher. study. institutions in the specialties "Microbiology", "Biology" and "Physiology".
6. The microbial mass should not contaminate hands, table and surrounding objects. The spilled microbial suspension is neutralized using disinfectants.
7. After the end of the work, the cultures are handed over to the teacher, the inoculated test tubes and cups are placed in a thermostat.
8. Bacteriological loops, needles, tweezers and other metal objects, after contact with microorganisms, are burned in the flame of an alcohol lamp and placed in a special stand.
9. Used slides and coverslips, pipettes, spatulas, etc. are placed in a 3-5% solution of carbolic acid or other disinfectant solutions.
10. Spent cultures in test tubes, Petri dishes, etc. are neutralized with disinfecting solutions for 24 hours, then the dishes are boiled and washed.
11. Personal hygiene should be strictly observed - after finishing work, hands should be thoroughly washed with soap and water.
12. It is necessary to observe safety precautions when working with electrical appliances and chemical reagents.
GENERAL MICROBIOLOGY
Chapter I. Microscope and microscopy technique
1. Light-optical microscopy
2. Dark field microscopy
3. Phase contrast microscopy
4. Luminescence (fluorescence) microscopy
Chapter II. General understanding of cultivation, seeding technique and the necessary equipment for working with microorganisms
Chapter III. Methods for the preparation of preparations of microorganisms
Chapter IV. Microbial cell research
1. Forms of cells of microorganisms
2. The structure of cells of microorganisms (cytochemical research methods)
3. Gram staining of cells of microorganisms
4. Coloration of spores in bacteria
5. Coloring of capsules
6. Coloration of flagella
7. Coloring of the nuclear substance of bacteria
8. Coloring of inclusions of cells of microorganisms
Chapter V. Nutrition of microorganisms
1. The importance of individual nutrients
2. Preparation of culture media
3. Methods of sterilization
Chapter VI. Counting the number of bacteria and isolating a pure culture
1. Counting the number of bacteria in the soil
2. Determination of the qualitative composition of bacteria
3. Counting the number of microorganisms in water and other liquids
4. Counting the number of bacteria in the air
5. Isolation of pure cultures of bacteria
Chapter VII. Determination of the type of bacteria
Chapter VIII. Conversion of nitrogen-free organic substances by microorganisms
Fermentation processes
1. Alcoholic fermentation
2. Lactic acid fermentation
3. Butyric acid fermentation
4. Fermentation of pectin substances
5. Fermentation of cellulose
6. Oxidation of fiber
7. Oxidation of fat
8. Oxidation of hydrocarbons
Chapter IX. Conversion of organic and mineral nitrogen compounds by microorganisms
1. Ammonification
2. Nitrification
3. Denitrification (nitrate respiration)
4. Biological fixation of atmospheric nitrogen
Chapter X. Transformation of Sulfur, Iron and Phosphorus Compounds by Microorganisms
1. Conversion of sulfur compounds by microorganisms
2. The participation of microorganisms in the conversion of iron
3. Conversion of phosphorus compounds by microorganisms
AGRICULTURAL MICROBIOLOGY
Chapter XI. General microbiological analysis of soil
1. Research methods
2. Groups of microorganisms, composition and preparation of culture media
3. Taking an average soil sample and preparing the sample for microbiological analysis
4. Preparation of soil suspension
5. Consideration of various groups of microorganisms
6. Determination of the total number of microorganisms in the soil by direct counting under a microscope
Chapter XII. Study of microorganism cenoses
1. Glass fouling method according to N.G. Kholodny
2. Study of microbial cenoses in soil by the method of Perfiliev and Gabe
3. Perfiliev capillary method modified by Aristovskaya
4. Identification of microorganisms of the autochthonous group participating in the decomposition of humic substances, according to the Vinogradsky method in the Tepper modification
5. Identification of microorganisms participating in the decomposition of humic substances by the Tepper method
Chapter XIII. Determination of the biological activity of the soil
1. Determination of the biological activity of the soil by the intensity of decomposition of the canvas (method of Mishustin, Vostrov and Petrova)
2. Determination of the general microbiological activity of the soil for the release of carbon dioxide
3. Determination of the ammonifying activity of the soil
4. Determination of the ammonifying activity of microorganisms
5. Determination of the nitrifying activity of the soil
6. Determination of denitrifying soil activity
7. Determination of nitrogen-fixing activity of microorganisms
Chapter XIV. Study of bacteria in the root zone of plants and on roots
1. Counting bacteria in the rhizosphere using the Krasilnikov method
2. Consideration of rhizosphere and root microflora by the method of successive root washing according to E. 3. Tepper
3. Isolation of pure cultures of nodule bacteria, quantitative accounting in the soil, determination of their activity and virulence
Chapter XV. Analysis of bacterial preparations
Chapter XVI. Microbiology of feed
1. Epiphytic microflora of grain and its change during storage of feed
2. Silo analysis
3. Yeast feed
Chapter XVII. Microflora of milk and dairy products
1. Bacteriological analysis of milk
2. Methods for isolating lactic acid bacteria in a pure culture
3. Acquaintance with the microflora of butter
Literature Index
Transcript
1 Ministry of Education and Science Russian Federation Federal Agency for Education Moscow State University of Environmental Engineering Kustova N.A. LABORATORY PRACTICE IN MICROBIOLOGY Moscow 2005
2 Laboratory workshop in microbiology is intended for students of specialties 3207 and 3302 in the discipline "Fundamentals of Microbiology and Biotechnology", as well as for students of the Department of Environmental and Industrial Biotechnology in the discipline "Environmental and Industrial Microbiology". The workshop is divided into three sections. The first section is devoted to issues of general microbiology. In the works of this section, the morphological structure of different groups of microorganisms, methods of microscopic examination, the technique of microbiological inoculation, methods of sterilization and methods of quantitative accounting of microorganisms are studied. The second section contains works on the use of microbes in biotechnology to obtain various substances of organic acids, alcohols, antibiotics, enzymes. In the works of the third section, questions of ecological microbiology are studied. Some of the works show the role of microorganisms in global biogeochemical cycles, and the rest are devoted to the problems of biotechnological environmental protection. Each topic contains a theoretical introduction and a practical part, which describes the methods used, the procedure for performing the work, the content of the report on the work, as well as control questions. 2
3 FOREWORD The laboratory workshop on microbiology is intended for 3rd year students of specialties 3207 and 3302 in the discipline "Fundamentals of Microbiology and Biotechnology", as well as for 4th year students of the Department of Environmental and Industrial Biotechnology, specialization "Biotechnological protection of the environment" in the discipline "Environmental and industrial microbiology ". The laboratory workshop is based on "Methodological instructions for laboratory work", ed. PI Nikolaev, which were used at the department "Processes and devices of microbiological production" since the creation of the department. Senior researcher, Ph.D. N.V. Pomortseva. Methodological guidelines were prepared under her leadership academics departments: M.A. Boruzdina, I.E. Lomova, N.A. Kustova, T.A. Makhotkina and K.A. Solovieva. The change curriculum in accordance with the new specialty environmental engineer has caused the need to expand the course of microbiology and supplement it with tasks on biotechnological methods of environmental protection. The laboratory workshop is divided into three sections. The first section is devoted to general microbiology: the morphology of microorganisms, methods of its study, the technique of microbiological crops, methods of quantitative accounting of microorganisms. The second section covers some examples of the use of microbes in industry. The third section covers the issues of the ecology of microorganisms, their role in global cycles of elements, as well as in biotechnological methods of environmental protection. The post-graduate student of the department N.V. Zyabreva and senior researcher took part in the preparation of this workshop. E.S. Gorshina. The author expresses his deep gratitude to Assoc. department Microbiology MSU N.N. Kolotilova for valuable comments and advice, as well as senior researcher. P.P. Makeev for his help in formatting the text and illustrative material. 3
4 4 GENERAL RULES FOR WORK IN A MICROBIOLOGICAL LABORATORY Rules for work and behavior in a laboratory Rules for work and behavior in microbiological laboratory have a lot in common with the rules of work in chemical laboratories, but have their own specifics. In most cases, a microbiologist works with pure cultures of microorganisms, i.e. with microorganisms of any one genus, species and strain. Since there are foreign microbes on all surrounding objects and in the air, special work methods are used to avoid contamination of the studied culture of the microorganism or the person himself. For this, culture media, dishes, instruments are sterilized, the laboratory and workplaces are kept clean, and certain rules are observed when working with microbes. There should be no unnecessary items in the laboratory. Wet cleaning should be done regularly. Various surfaces of laboratory rooms are periodically disinfected. Disinfection is disinfection, i.e. destruction of pathogens of infectious diseases at sites external environment... To do this, use a 0.5 to 3% solution of chloramine or a 35% solution of phenol (carbolic acid). The work table should be disinfected with a 70% solution of ethyl or isopropyl alcohol. Air disinfection is achieved by simple ventilation (at least min). A more effective way to disinfect the air is to irradiate a room with ultraviolet rays using germicidal lamps. Ultraviolet irradiation is especially often used to sterilize a box. Boxing is a special small room for inoculation of pure cultures, quantitative registration of microorganisms on Petri dishes and some other work requiring particularly clean conditions. Before work, the box is irradiated for min. The table is wiped with alcohol, the walls and floor are periodically washed. Instead of a box, laboratories can be equipped with laminar flow cabinets (Fig. 1), which are also sterilized with bactericidal lamps. At
During operation, a fan is turned on to create a laminar flow of sterile air passed through bactericidal filters. The main equipment of the microbiological laboratory includes: microscopes, thermostats for growing microorganisms, sterilization equipment (autoclave and drying cabinet), centrifuges, a distiller, a refrigerator for storing museum cultures of microorganisms, cabinets for placing glassware and reagents, necessary instruments (photoelectric colorimeters, pH -meters, etc.). Each student is assigned a workplace where they place: a microscope covered with a cover, a bacteriological loop, slides and cover glasses, sterile pipettes, an alcohol burner, strips of filter paper, a marker on the glass, a vessel with a disinfectant liquid. There should be nothing on the table that is not directly related to the performance of the work. In laboratory classes in microbiology, safety rules must be followed. 5
6 6 Brief information on safety in the laboratory In microbiological practice, glassware made of chemical glass is widely used. Care must be taken when working with it. Carefully remove shards of broken dishes. In analyzes, strong solutions of alkalis and acids are often used. You need to work with them with great care, since these substances are harmful to the skin of the hands and clothes. If acid is accidentally spilled, it should be covered with a large amount of soda and then rinsed several times with water. The spilled alkali must be thoroughly wiped off, and the objects on which it has come into contact must be treated with a weak solution of acetic acid. If acids or alkalis come into contact with human skin, they must be washed off immediately with plenty of water. The microbiological laboratory deals with living microorganisms. The main work is carried out sterile, i.e. work with one culture of microorganisms, which should not be contaminated with foreign microbes. To prevent contamination of crops, special sterilization methods are used. It is also important to keep the laboratory clean. Crockery containing cultures of microorganisms should not be left open. The biomass of microorganisms, if it is not needed for analysis, is thrown away only after sterilization in an autoclave. Crops of microorganisms are produced near the flame of a gas or alcohol burner, so you should beware of burns, and above all, carefully pick up long hair. The burner should only burn when needed. If, during sowing, a cotton plug, alcohol lamp or paper accidentally catches fire, extinguish the fire with a towel. For larger fires, fire extinguishers are used. Used pipettes, slides, coverslips, spatulas, etc. placed in a vessel with a disinfectant liquid. Students must constantly remember that they are dealing with microorganisms that may not always be safe, especially in work on the isolation of microbes from environmental objects. Therefore, at the end of the lesson, students should tidy up the workplace and wash their hands with soap and water.
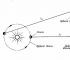
7 In the event of a power failure, turn off electrical appliances and their centralized power supply. SECTION 1. GENERAL MICROBIOLOGY TOPIC 1. The morphology of microorganisms and methods of studying it Microbiology studies organisms that are microscopic in size, ie. measured in micrometers or fractions of micrometers. One micrometer is equal to 10-6 meters and is abbreviated as microns. Microorganisms are characterized by intensive metabolism and are capable of carrying out a variety of chemical transformations. Different microorganisms differ both in their structure and in the biochemical processes that they carry out. Combining them into one group is caused not only by their small size, but also by the commonality of methods of cultivation and research. To study the structure of microorganisms, their appearance, shape, size, i.e. to study the morphology of microorganisms, use a microscope. The smallest particles that can be seen in modern light microscopes are more than 1/3 of the wavelength of light, i.e. not less than 0.2 microns, which is associated with the use of the visible part of light having a wavelength from 0.4 microns to 0.7 microns. Microscope device Fig. 2 shows the appearance of the microscope MBI-3, which is widespread in research and educational practice. The examined object, the preparation is placed on a stage and illuminated from below by light rays that come out of the illuminator, fall on the mirror, then pass through the condenser and focus on the preparation. The main parts of the microscope: eyepieces, a tube, a revolving attachment with objectives, a stage with clips for preparations, a condenser, macro and microscrews for focusing and, finally, a tripod in which all this is mounted. 7
8 Before microscopy, check the correctness of the lighting installation (according to Koehler). To do this, by moving the lamp holder with a light bulb, they achieve a clear image of the thread 8
9 incandescence of the lamp on the fully closed diaphragm of the condenser so that this image completely fills the hole of the condenser. Having closed the diaphragm of the illuminator, open the diaphragm of the condenser and, moving the condenser, achieve a sharp image of the diaphragm of the illuminator in the field of view of the microscope. So that the bright light does not blind the eyes, the filament of the lamp is first reduced. And finally, the image of the aperture hole is set in the center of the field of view, and the diaphragm of the illuminator is opened so that the entire field of view is illuminated. The microscope is an optical system with two stages of magnification: the first magnification is carried out by the objective lens, the second by the eyepiece. The lens gives a magnified reverse image of the object, which is viewed through the eyepiece. As a result, the eye of the observer sees a highly magnified reverse image of the object. Therefore, the movement of the object to the left is perceived by the eye as movement to the right. The overall magnification of the microscope, i.e. The magnification at which an object is viewed under a microscope is defined as the product of the magnifications of the objective and the eyepiece. Objectives give a magnification of 10, 40, 60, 90 times, eyepieces at times. If a binocular attachment is used, it provides additional magnification. In fig. 3 shows a schematic diagram of the optical system of the microscope. Objective O forms in plane Z a real inverted image A of object A. The image given by the lens is enlarged further with the help of eyepiece E. Since Z is in the focus of eyepiece E, the observer sees an enlarged virtual image A in plane X, which is usually located 25 cm from the eye , i.e. at a distance most convenient for near vision. It should be borne in mind that such an idea of the mechanism of image formation is in the highest degree simplified, because it ignores the influence of diffraction and a number of other factors. In working with a microscope, students study preparations of microbes with a magnification in times. The highest magnification given by an optical microscope is 3000 times. The smallest particle size that can be viewed in such a microscope, 9
10 is equal to 0.2 μm, which is due to the wavelength of the visible part of the spectrum. Morphology of microorganisms The world of microorganisms includes a huge variety of forms that do not constitute a single systematic group. The main objects of microbiology are bacteria, but besides them, microbiologists also study yeast, fungi, microscopic algae and some protozoa. In fig. 4 shows the main groups of microorganisms (with the exception of protozoa); the proportions of their sizes are preserved. All living organisms, except viruses, have cellular structure... In accordance with their cellular organization, they are divided into prokaryotic and eukaryotic. ten
11 The main difference between eukaryotes and prokaryotes is the presence of secondary cavities that separate the nucleus and other cellular structures from the cytoplasm. It was the appearance of secondary cavities that made it possible to make a leap in the evolution of the entire living world due to an increase in the inner surface of eukaryotic membranes. This made it possible, in accordance with the increase in the diffusion rate, to simultaneously carry out a greater number of biochemical reactions occurring on the membranes. Prokaryotes are bacteria, including actinomycetes and cyanobacteria. Eukaryotes are all plants, animals, yeast, fungi, protozoa. Among prokaryotes, a group of archaebacteria is currently distinguished, which includes methanogens, extreme halophiles, (living in very salty water) extremely thermophilic bacteria that oxidize and reduce molecular sulfur, as well as thermoplasmas, devoid of a cell wall. The new division was made based on the comparison of nucleotide sequences in small fragments of ribosomal RNA. eleven
12 Archaebacteria differ in the composition of cell walls, lipids and some other physiological and biochemical features (for example, they have a different mechanism for fixing CO 2). Thus, in the structure of the cellular organization, three groups are currently distinguished: archaea (according to the new nomenclature of Archaea, archaea), eubacteria (according to the new nomenclature of Bacteria, bacteria), and eukaryotes (according to the new nomenclature of Eukarya). Work 1. Microscopic study of bacteria Morphology of bacteria Theoretical introduction This group of microorganisms is the most numerous, widespread in nature and is of great industrial importance. For the naming of microorganisms, a binary nomenclature is used, as in zoology and botany. In accordance with this nomenclature, each species has a name consisting of two Latin words. The first word means genus and the second kind. The generic name is always written with a capital letter, and the specific name with a lowercase letter. Most bacteria unicellular organisms spherical, rod-shaped or convoluted. There are a small number of filamentous forms among bacteria. The bacteria are very small, the diameter of the cell of the globular bacteria is 1 2 µm. Bacteria multiply by division (under favorable conditions, division occurs in minutes). Some bacteria are mobile. The ability to move is associated with the presence of special organelles of the flagella. The most simple in shape are spherical bacteria (cocci). They meet either in the form of single balls, or balls interlocked with each other. According to the location of cells after division, spherical bacteria are divided into monococci (single cocci), tetracocci (combined by 4), sarcins (combined by 8), staphylococci (bunches), streptococci (chains of cocci). 12
13 Rod-shaped bacteria are the largest group of bacteria. They have a cylindrical cell shape with rounded or pointed ends and vary greatly in length to width. They can be located singly or form short or long chains. Rods can be of various lengths, usually a few microns, and a width of about 1 micron. Some rod-shaped bacteria form special spore bodies inside the cell. Each cell forms one spore, which serves to endure adverse conditions. The spore germinates under appropriate conditions (temperature, humidity, nutrients), turning into a stick. The resistance of bacterial spores is superior to that of any living organism. For example, the spore of the hay bacillus Bacillus subtilis can withstand a temperature of 100 ° C for 3 hours. This spore resistance makes it difficult to fight infections. Twisted microorganisms differ in the degree of curvature of the cells and in the number of turns. They are divided into vibrios, spirillae and spirochetes. If a bacterium has one incomplete curl of a spiral, then it is called a vibrio. If the bacterium has several spiral curls, then it is called spirilla, and microbes that have a curled shape with a large number of small curls are called spirochetes. Filamentous bacteria are filaments made up of cylindrical or disc-shaped cells. Some types of threads are enclosed in a mucous membrane, which can be impregnated with iron hydroxide or manganese salts. The accumulation of heavy metals from solutions occurs in the cells of some iron bacteria. Large filamentous bacteria p. Beggiatoa deposit sulfur in their cells. Filamentous bacteria usually live in sea and fresh waters, they are also found in decaying organic residues, in the intestines of animals. Cyanobacteria include a large group of organisms that combine the prokaryotic structure of a cell with the ability to carry out photosynthesis. Cyanobacterial cell pigments, in addition to chlorophyll a (green), contain phycocyanin pigment 13
14 blue. For this reason, they were previously called blue-green algae. Most of them are multicellular organisms, which are long, most often unbranched filaments (trichomes). The cells in the filaments are united by a common outer wall. Sometimes mucous accumulations of "mats" form. Reproduction is carried out by breaking down the thread into separate sections. Some species move by sliding (p. Spirulina). Actinomycetes (branching bacteria, radiant fungi) are a large group of prokaryotic microorganisms that form thin branching filaments several mm long and 0.5–1.5 µm in diameter. They are a peculiar group of microorganisms, which morphologically bears a resemblance to molds (Fig. 5). The cells of a significant part of the representatives of this group are capable of branching, which is a characteristic feature of fungi. However, the length of the branching filaments of actinomycetes reaches several millimeters, while the length of the mycelium of fungi is several centimeters. The hyphae of fungi are usually several times thicker than the filaments of actinomycetes. According to morphology and development, actinomycetes are divided into higher and lower forms. The highest are organisms with a good 14
15 developed septic or non-septic mycelium and special sporulation organs. Spores are formed in the form of chains on special spore-bearing hyphae of the aerial mycelium. The structure of the sporulation organs is different in different species: long or short, straight or spiral (Fig. 6). By the presence of mycelium and the structure of sporulation organs, higher actinomycetes resemble filamentous fungi. Some actinomycetes have mycelium only in a young culture, which disintegrates with age to form rod-shaped and coccoid cells. The lower forms of actinomycetes do not have true mycelium. The ability to form mycelium is expressed in them only in the tendency of cells to branch. The lower actinomycetes include, for example, species of the genus Mycobacterium, which have the ability to change the shape of cells with the age of culture (Fig. 7). This property is called pleomorphism. Among the higher actinomycetes, species of the genus Streptomyces occupy the leading place in terms of abundance in natural environments. Actinomycetes play an important role in 15 processes
16 soil formation and creation of soil fertility. Actinomycetes destroy complex organic compounds (cellulose, humus, chitin, lignin, etc.) that are inaccessible to many other microorganisms. Almost all species of the genus Streptomyces form specific waste products with antibiotic properties. Some species are causative agents of diseases of plants, animals and humans. In addition to the main forms of bacteria, there are stem and budding bacteria that carry outgrowths, called prostheses. (fig. 8) 16
17 The functions of the stitches are different. In some bacteria, they serve for reproduction, in others for the attachment of the cell to the substrate. 17
18 18 Practical part The purpose of the work is to study the morphology of representatives of bacteria Procedure for performing the work Performing the first work, students learn how to use a microscope, look at the finished preparations of representatives of bacteria, then independently prepare preparations of living bacteria and microscop them. First, students microscop the finished fixed preparations of bacteria. Fixed preparations are microorganisms suspended in an aqueous medium, dried on a glass slide and stained with aniline dyes. Students prepare preparations of some living microorganisms on their own. To do this, a small drop of tap water is applied to a clean glass slide, into which a small amount of the studied microbes is introduced with a calcined and cooled bacteriological loop, thoroughly stirred and covered with a cover glass. Excess water is removed with filter paper. The specimen is placed on the stage and secured with clamps. First, looking through the eyepiece and rotating the macroscrew, they achieve a sharp image of the object at low magnification with a 10x objective. Then the microscope is transferred to high magnification with a 40x objective. Rotation of the screws must be done with caution, as if the lowering is too sharp, the lens can be crushed by the objective. When microscopying, it should be borne in mind that the microscope, especially at high magnifications, does not capture the entire depth of the object, therefore, when the tube is gradually lowered with the help of a microscrew, the object is first seen from above and then in an optical section. 1. Viewing fixed preparations of microorganisms. Lactococcus lactis lactic acid fermentation agent; the shape of the cells is spherical cocci; cells are connected in chains. It is used to obtain lactic acid products.
19 Lactobacillus acidophilum rod-shaped bacteria; the causative agent of lactic acid fermentation. It is used to obtain lactic acid products. Acetobacter aceti are rod-shaped bacteria that oxidize ethanol to acetic acid. Used to make edible vinegar. Streptomyces griseus actinomycete, cell shape in the form of thin branched filaments; producer of the antibiotic streptomycin. Saccacharopolyspora erythrae actinomycetes, cell shape in the form of thin branched filaments; producer of the antibiotic erythromycin. 2. Viewing live preparations of Bacillus subtillis cells form in the form of thin movable rods; forms spores; producer of enzyme preparations. Spirulina platensis cyanobacteria; the shape of cells in the form of a single filament, consisting of cylindrical cells, tightly adjacent to each other; has a sliding motion. It is used as a food additive. Contents of the report 1. Latin name of the microorganism. 2. Cell morphology (give a drawing showing the magnification of the microscope and keeping the cell size ratio). 3. Use of the studied bacteria in industry. Control questions 1. Describe the microscope design. 2. How to determine the magnification of a microscope? 3. What is the shape of bacteria cells. 4. Name the features of the morphology of actinomycetes. 5. What representatives of bacteria do you know and what is their practical significance? Work 2. Morphology of eukaryotic microorganisms Theoretical introduction 19
20 Eukaryotic microorganisms studied by microbiologists include fungi, yeast, microalgae and some protozoa. Morphology of fungi Fungi are chlorophyll-free microorganisms with filamentous cells. The long, branched filaments they form are called hyphae. The hyphae together form the mycelium. In terms of their size, fungal hyphae are much larger than actinomycetes. The mycelium in a number of molds is divided by septa (septate mycelium), while other types of septa are absent. In biotechnology, molds are mainly used as producers. Some representatives of phycomycetes, marsupials and imperfect mushrooms are called "mold fungi". On dense substrates, molds form round, fluffy, cobweb-like, cotton-like or powdery colonies of green, yellowish, black or white color. Mold colonies consist of a large number of hyphae. Most of the hyphae develop in the air, and some in the thickness of the substrate. Conidiophores are often formed on hyphae, on which spores are formed either inside the sporangium or in the form of exospores arranged in chains. Conidiespores, or conidia, serve to asexual reproduction(fig. 10). Conidia, getting into favorable conditions, germinate into the mycelium. Molds are very widespread in nature and have a powerful enzymatic apparatus. Therefore, they are the main destructors of organic compounds in nature. Molds are also widely used in industry for the production of organic acids, antibiotics and enzymes. twenty
21 Yeast morphology Yeast is a separate group of unicellular microscopic fungi of great practical importance. Yeast cells are large round or oval cells (Fig. 11). Some yeast can form a rudimentary mycelium called pseudomycelium. Cell size ranges from 3 µm to 10 µm in length and 2 to 8 µm in width. Most yeasts reproduce by budding. Moreover, at 21
On the surface of the cell, a small bulge of the kidney appears (sometimes not one, but several), gradually increasing in size and, finally, separating from the cell producing it. The kidney separated from the mother cell becomes a new yeast cell. Some yeast multiply by division. Large vacuoles are clearly visible in the fine-grained contents of live yeast (protoplasm). Vacuoles are cavities inside the protoplasm filled with cell sap. It consists of electrolytes, proteins, carbohydrates and enzymes dissolved in water. In young yeast cells, the protoplasm is homogeneous, and at later stages of development, vacuoles appear in the protoplasm filled with cell sap with metabolic products. When the nutrient medium is depleted, sporulation occurs in many yeasts. In some yeast species, the spores are round and covered with a smooth shell. In favorable conditions, spores germinate. Yeast is widespread in nature. They are found on grapes, other berries and fruits, milk, water and soil, and human skin. Many yeasts carry out alcoholic fermentation and are used for the production of alcohol in bakery, winemaking, and brewing. 22 Protozoa morphology Protozoa are unicellular organisms of the animal world. Among microorganisms, they are the most complexly arranged, having primitive organs characteristic of multicellular animals. Some of them have oral and anal openings, contractile and digestive vacuoles. Protozoa reproduce asexually (cell division) and sexually. The classification of protozoa is based on the modes of movement. 1. Sarcodes. They move and grab food using pseudopodia, or false legs. Amoeba is a typical representative. The size of amoebas does not exceed microns.
23 2. Flagellates. They have a dense plasma membrane and move with the help of flagella. Soil forms of flagella are very small (2 5 µm), and water forms are large (up to 20 µm). The typical representative is Euglena. 3. Ciliary. The most highly organized protozoa. Cell sizes range from 20 to 80 microns. A typical representative is the ciliate-shoe Paramecium, which is cultivated for feeding fish fry. 4. Sporozoans. Fixed forms. Many pathogens, such as Plasmodium malaria. Protozoa are widespread in nature. They are found in water bodies, in silt and soil. The meaning of protozoa in nature is very diverse. They live in the gastrointestinal tract of various animals, taking part in the digestion of plant food, participate in the mineralization of organic residues in the soil, and are also an important part of the biocenosis in sewage treatment plants. Feeding on bacteria and suspended solids, they help to clarify the water. The simplest ones perform the function of indicators: by the development of certain forms, one can judge the quality of wastewater treatment. So, the predominance of amoebas and the absence of ciliates in the activated sludge indicates the poor performance of treatment facilities. The ciliate Tetrahymena is widely used for initial assessment toxicity. Various types of protozoa are shown in Fig. 12. Morphology of algae Algae are a large group of plant organisms. The common for all of them the presence of chlorophyll and the resulting photoautotrophic nutrition, the ability to synthesize organic matter using energy sunlight and carbon dioxide. In many algae, the green color of chlorophyll is masked by other pigments. Among them, there are very small unicellular and multicellular forms attributed to microorganisms, as well as 23
24 multicellular organisms that live in the seas and oceans and sometimes reach gigantic sizes .. The objects of study of microbiology are some algae of microscopic size. They have a variety of forms and live both on land and in the aquatic environment (Fig. 13). 24
25 Practical part The purpose of the work is to study the morphology of representatives of various groups of eukaryotes. Procedure for performing the work Students independently prepare live preparations of representatives of fungi, yeast, algae and protozoa; they are microscoped with a x40 objective and sketched with an indication of the magnification of the microscope. Aspergillus niger molds form cells in the form of a mycelium with septa; some hyphae have unbranched conidiophores with spores. Conidiophores are unicellular, globularly swollen; on the surface of the swelling, there are short pin-shaped cells (sterigmas), each of which detaches along a chain of black-colored conidia. It is used to obtain organic acids and enzymes. Penicillium chrysogenum hyphae have septa; on some hyphae there are branched conidiophores with spores. Conidia are formed at the ends of whorly branched conidiophores. It is used to obtain the antibiotic penicillin. 25
26 Yeast Saccharomyces cerevisiae solitary yeast with oval and round cells; reproduce by budding. They are used in brewing, baking, alcohol production. In nature, they are found on the surface of berries and other fruits. Saccharomyces vini cells are oval and round; reproduce by budding; after budding, they do not separate for some time, forming small "twigs". They are used in winemaking. Rhodotorula glutinis cells are elliptical; cells are single; reproduce by budding. They are colored orange due to the content of carotenoids in the cells. Can grow in environments with petroleum hydrocarbons as a carbon source. Used as feed yeast. Candida tropicalis yeast with oval and highly elongated cells, forming a "pseudomycelium". Can grow in a mineral environment with hydrocarbons as a carbon source. Used as feed yeast. On an industrial scale, they are grown on waste from the production of alcohol and paper. Algae Chlorella vulgaris is a microscopic green alga with rounded cells (5-10 microns in diameter); reproduce by autospores, which are formed inside the mother cell in an amount from 4 to 32 and are released after the rupture of its membrane. They can be used for mass cultivation in order to obtain fodder protein, as well as for air regeneration in closed systems (submarines, space stations etc.). Scenedesmus sp. belongs to the group of green algae; has an oval cell shape; outer cells are often pointed; cells are connected together in four. It is used to obtain food protein. 26
27 Protozoa The preparation is prepared from the activated sludge of the aeration tank. Study the morphology of the found protozoa and sketch them. Contents of the report 4. Latin name of the microorganism. 5. Cell morphology (give a drawing showing the magnification of the microscope and keeping the cell size ratio). 6. Use of the studied microorganisms in industry. Control questions 1. Describe the shape of cells of representatives of fungi and their practical use. 2. Describe the shape of yeast cells; name representatives, and tell about their practical use. 3. Tell us about the morphology of microalgae and their practical use. 4. Name the representatives of the simplest and tell about their application in biotechnology. Work 3. Methods for studying the morphology of microorganisms The most common method for studying the morphology of bacteria is microscopy of fixed preparations prepared from pure cultures of microorganisms or from a test sample. The study of microorganisms in a living state is used in the study of larger forms and the observation of cell motility. Preparation of fixed preparations of microorganisms To prepare fixed preparations of microorganisms, a smear is first prepared, dried, fixed, and then stained. The smears are prepared on perfectly clean glass slides. You can degrease glasses with ethyl alcohol, a mixture of equal volumes of alcohol and ether, and other 27
28 liquids. The easiest and most convenient way to degrease glasses is to wipe them on both sides with a piece of laundry soap. The soap is removed from the glass with a piece of dry cotton wool or a napkin. When making a smear from a colony of bacteria grown on an agar medium, a drop of water or saline is first applied to a defatted glass. Then the bacteriological loop is ignited in the burner flame. After that, after cooling the loop on the inner wall of the tube, a part of the colony without agar is captured with a loop. The culture loop is placed in a drop of water or saline on a glass and 2 3 circular movements are made. Some of the bacteria are suspended in the liquid. Excess bacteria remaining on the loop are burned in the burner flame, heating the loop to red hot. Then, with a cooled loop, smear a drop of the suspension on the glass. The area of the smear should be 1.5-2 cm in diameter. The smear should be thin with an even distribution of material. If a smear is prepared from a culture grown in a liquid nutrient medium, then, observing the same rules of sterility, take a drop of the culture with a loop or pipette and apply it to the middle of a defatted glass. The pipette with the remainder of the culture is immersed in a vessel with a disinfectant solution. The drop is evenly distributed over the glass with a bacteriological loop. The loop is burned in a burner flame and placed in a tripod or glass. The smear is dried in air or in a stream of warm air, holding it by the longitudinal ribs high above the burner flame. The boundaries of the smear are outlined with a wax pencil on the back of the glass, and on the side of the smear, at one of the ends of the glass, the number of the preparation is put. By these marks, you can easily navigate in the location of the smear on the glass. The dried smear is fixed. Fixation has the following goals: 1) to kill germs, which makes the drug safe for further work; 2) attach microbes to the glass so that they are not washed off during painting and rinsing with water; 3) improve paint receptivity. 28
29 The simplest method, suitable for almost all microbiological objects and the most widespread method in practice, is fixation in the burner flame. For this, the slide is passed 3-4 times through the hottest upper part of the burner flame, avoiding excessive overheating of the preparation, so as not to cause protein denaturation and disruption of the structure and morphology of bacteria. Distinguish between simple and complex differential painting methods. Simple staining is used to detect microbes in the test material, to determine their number, shape and location. Simple dyeing consists in applying a single aniline dye to the preparation. Most often, red fuchsin is used for these purposes, as well as an alkaline solution of methylene blue (Leffler's blue) blue. Staining technique: a well-fixed specimen is placed with a smear upwards on a glass bridge above the bath. One of these paints is applied to the surface of the smear with a pipette or dropper. Fuchsin is kept on a smear for 1 to 3 minutes, and blue for 3 to 5 minutes. The paint is drained from the smear, the preparation is washed with water, dried in air or carefully blotted with filter paper. A drop of immersion oil is applied to the dried smear, the preparation is placed on the stage of the microscope and microscoped with an immersion objective (90) in transmitted light. Differential methods of staining bacteria. Sophisticated staining techniques are essential in identifying and differentiating different types of microbes. They are based on the peculiarities of the physicochemical structure of the microbial cell and are used for a detailed study of the structure of the cell and the identification of distinctive features in relation to some dyes. With these methods, the smear is stained with several paints and additionally treated with mordants or decolouring agents with alcohol, acid, etc. These methods include the most important staining method for differentiating bacteria, Gram staining. This method reveals 29
30 the ability of bacteria to retain dye or discolor in alcohol, which is related to the chemical structure of the cell wall. According to the structure of the cell wall, all bacteria are divided into two groups: 1) gram-positive staining according to Gram and 2) gram-negative ones not staining according to Gram. The attitude to Gram staining is such an important differential characteristic of bacteria that it is necessarily mentioned when characterizing them and serves as a taxonomic feature. 30 Gram stain technique A strip of filter paper previously impregnated with gentian violet is placed on the fixed smear. Apply 3-5 drops of tap water to the strip. After 1-2 minutes, the paper is removed with tweezers, and Lugol's solution is poured onto the preparation. After 30 sec and 1 min, the Lugol's solution is poured off. A few drops of 96 O alcohol are applied. Discolored for 1 min until the disappearance of grayish-violet streaks. The preparation is washed with water. Fuchsin is poured onto the smear and kept for 1 2 minutes. The paint is drained, the preparation is washed with water, dried with filter paper. Microscopic examination with an immersion system. Gram-positive bacteria are colored violet-blue (for example, the butyric acid fermentation rod Clostridium pasteurianum), and gram-negative bacteria turn pink-red (E. coli Escherichia coli). In addition to this traditional Gram staining technique, there is a quick and easy method for Gram differentiation without staining. Bacterial cells (preferably 1-2 days old) are placed in a loop in a drop of 3% KOH on a glass slide, stirred in a circular motion, and after 5 to 8 s the loop is sharply raised. The suspension of gram-negative bacteria becomes viscous and stretches behind the loop, forming mucous cords. Gram-positive bacteria are evenly distributed in the droplet of alkali (as in water). The reaction is considered negative if the formation of mucous cords is not observed within 60 s. The increase in viscosity is caused by lysis of the cell walls of gram-negative bacteria in solution
31 alkali and DNA release. The method of bacterial differentiation according to Gram without staining should be used only for preliminary diagnostics, or for calculating the approximate ratio of colonies of gram-positive and gram-negative bacteria. Detection of living and dead cells by methylene blue staining The number of living and dead cells can be determined by staining with methylene blue solution. The method is based on the different permeability of living and dead cells of microorganisms. Cell permeability in dead cells is impaired, so the dye freely passes through the cytoplasmic membrane and is adsorbed by protoplasm. Under the microscope, dead cells appear blue, living cells are colorless or pale blue. Coloring is carried out as follows: a drop of a 2% solution of methylene blue is applied to a glass slide, covered with a cover glass, the excess paint is drawn off with a piece of filter paper. The drug is viewed under a microscope, in 10 fields of view, the number of living and dead cells is counted; the number of dead cells is expressed in%. Example: the average number of living cells in one field of view is 10, the average number of dead cells in one field of view is 5, total number cells in one field of view of cells 100% 5 cells X X 33.3% 15 Thus, the number of dead cells in the studied suspension of microorganisms was 33.3%. Determination of cell sizes To determine the size of cells of microorganisms, it is necessary to have a special eyepiece with a scale (eyepiece micrometer) and a micrometer object. Ocular micrometer, at 31
32 in the simplest case, is a glass disc with a linear scale printed on it, which is inserted into the eyepiece (Fig. 14a). The micrometer object is a microscope slide, in the center of which is engraved a linear scale 1 mm long, with a graduation of 10 microns. Before measuring, it is necessary to determine the division value of the eyepiece micrometer for each magnification of the microscope. For this, the micrometer object is placed on the stage of the microscope and considered as a preparation; in this case, one of the visible divisions of the object-micrometer is aligned with the zero mark of the eyepiece micrometer scale and the coincidence of divisions of both scales is noted (Fig. 15). The number of ocular and objective divisions is counted in this segment, and the price of divisions of the ocular micrometer is calculated. If, after that, a microorganism preparation is placed on the stage instead of a micrometer object and examined 32
33 at the same magnification, it is possible to measure the size of the microbial cell using the scale of the eyepiece micrometer as a ruler. For accurate measurements, a special eyepiece micrometer with a sliding zero mark associated with the measuring drum is used (Fig. 14b). It allows you to determine the size of microorganisms with an accuracy of tenths of a micron. The zero mark is a double vertical line, the middle of which corresponds to the intersection of two thin lines in the form of a cross. Before measuring, it is necessary to know the scale division of the measuring drum. The reference object is a micrometer. Rotating the measuring drum, several divisions of the object-micrometer scale are circled by the zero mark. The vertical double line shows the number of complete revolutions of the measuring drum. Carrying out measurements of microorganisms, move the zero mark along the object and read the readings of the measuring drum. Practical part The aim of the work is to master the basic methods of studying the morphology of microorganisms. 33
34 Procedure for performing work 1. Prepare fixed preparations of lactic acid bacteria from biokefir and acidophilus. 2. Perform Gram staining of Bacillus subtilis (gram +) and Escherichia coli (gram-) cells. 3. Determine the size of the yeast cells of Saccharomyces cerevisiae. 4. Determine the number of living and dead Saccharomyces cerevisiae cells in the yeast suspension. 34 Contents of the report 1. The morphology of lactic acid bacteria cells (pictures of fixed preparations of lactic acid bacteria, indicating the magnification of the microscope). 2. Drawings of fixed preparations of gram-negative and gram-positive bacteria. 3. Determination of the scale interval of the measuring drum of the eyepiece micrometer. 4. Sizes of the yeast cell of Saccharomyces cerevisiae. 5. The number of living and dead cells in the yeast suspension. Test questions 1. How to prepare a fixed preparation of bacteria? 2. What is the reason for the difference in bacteria in Gram stain? 3. What is the Gram staining method? 4. How to determine the size of microorganisms? 5. How to determine the number of living and dead cells of microorganisms by staining with methylene blue? Topic 2. Cultivation of microorganisms: principles of compiling nutrient media; sterilization methods; methods of inoculation and reseeding of microorganisms. Classification and preparation of culture media To cultivate microorganisms means to artificially create conditions for their growth. For cultivation
35 microorganisms in the laboratory or in industry, nutrient media containing all the substances necessary for the vital activity of microorganisms are used. From the environment, nutrients enter the cell of the microorganism, and metabolic products are removed from the cell into the environment. For the life of microorganisms, water, carbon, oxygen, nitrogen, sulfur, phosphorus, potassium, calcium, magnesium, iron and trace elements are needed. All these substances must be contained in the nutrient medium. Even without one of them, there will be no growth at all, or it will be insignificant. Carbon, hydrogen, nitrogen, phosphorus and sulfur are called biogenic elements, since they account for about 90–95% of the dry mass of cells. Potassium, magnesium, calcium and sodium are called macronutrients, or ash elements. They account for up to 5-10% of the dry mass of cells. Iron, manganese, molybdenum, cobalt, copper, vanadium, zinc, nickel and some other cations of heavy metals are called trace elements and make up a fraction of a percent of the dry mass of cells. Highest value has carbon for any living organism. It is part of all organic molecules in the cell and accounts for about 50% of the dry biomass. In relation to carbon, all organisms are divided into autotrophic and heterotrophic. Autotrophs use carbon dioxide as a carbon source. Heterotrophs need ready-made organic compounds. Various organic substances can serve as a source of carbon for most microorganisms: proteins and their decomposition products, carbohydrates, fats, hydrocarbons. Nitrogen nutrition is in second place after carbon nutrition in terms of its value. Nitrogen is a constituent of amino acids and other cellular components that ensure the vitality of organisms. Nitrogen makes up 14% of the dry matter of cells. The source of nitrogen is nitrogen-containing organic or mineral compounds. Sources of mineral nitrogen are most often ammonium salts and nitrates. Proteins, amino acids, and nucleotides are used as organic sources of nitrogen. Some prokaryotes can use atmospheric nitrogen. 35
36 Phosphorus and sulfur are important cell biopolymers. Phosphorus (3% of dry matter of cells) is part of nucleotides and ATP, and sulfur (less than 1%) is part of some amino acids. As a source of phosphorus, phosphates are usually used, and sulfur is sulfates. Phosphorus and sulfur can also be used as organic compounds. For the growth of microorganisms, small amounts of macroelements are required: ions alkali metals(Na +, K +) and alkaline earth metals (Mg 2+, Ca 2+), which play an important role in the life of microorganisms. Macronutrients in microbial cells are necessary to regulate permeability, osmotic pressure, pH value. In addition to these metals, a number of trace elements (trace elements) are required for the growth of microbes. The mineral composition of the nutrient medium forms the distribution of electrical charges on the cell surface. Changing the electrical potential of cells can change their physiological activity. One of the main functions of trace elements is participation in enzymatic catalysis. Currently, the action of one fourth of all enzymes in the cell is associated with metals. In addition to the main components of the nutrient medium for the normal development of some microbes, additional substances are also needed, which are called "growth factors". Growth factors are the combined name of various chemical nature connections. These are mainly organic substances, the addition of which in very small quantities stimulates the growth and reproduction of microorganisms. They have the same meaning for microbes as vitamins are for higher organisms. Growth factors are mainly B vitamins, which play the role of regulators and stimulators of metabolism in microbes, or amino acids. Yeast autolysate, yeast extract, native proteins (blood, serum), etc. are used as growth factors. The nutritional requirements of microorganisms are very diverse. For this reason, there is no universal 36
37 medium, equally suitable for the cultivation of any microorganism. Depending on the goals of cultivation and the needs of a given microorganism, nutrient media differ in three ways: composition, physical condition and purpose. By composition, nutrient media are divided into two groups: 1) natural (natural); 2) artificial (synthetic). Natural environments are those that have an undefined chemical composition, since they include products of plant or animal origin, waste of various industries, containing organic compounds. Natural nutrient media provide intensive growth of a wide variety of microorganisms. They contain a rich array of organic and inorganic compounds, including all the necessary elements and additional substances. For example, the following culture media are most commonly used in laboratory settings. 1. Meat-peptone broth (MPB) extract from meat, contains products of incomplete breakdown of protein peptones, which contain organic carbon, organic nitrogen, phosphorus-containing, sulfur-containing organic substances. The BCH also contains all the minerals necessary for microorganisms. BCH is used in the cultivation of many types of bacteria. 2. Beer wort extract from sprouted barley grains, contains sugar (mainly maltose), nitrogenous substances, ash elements, various growth factors and B vitamins as a carbon source. Beer wort is a good environment for the development of many microorganisms, in particular yeast. mold fungi. Good natural media are milk, potatoes, fruit and vegetable teas. In industry, semi-synthetic or natural media are usually used. Very often, microbiological or microbiological waste is used as a carbon source. Food Industry: molasses (waste from sugar production), 37
Ministry of Agriculture of the Russian Federation FEDERAL FISHERIES AGENCY KAMCHATSK STATE TECHNICAL UNIVERSITY DEPARTMENT OF BIOLOGY AND CHEMISTRY CHEMISTRY AND MICROBIOLOGY OF WATER PREPARATION
Surname Code Name Workplace District Code Total: Tasks of the practical round of the regional stage of the XXXII All-Russian. BIOCHEMISTRY. EXTRACT IDENTIFICATION Equipment: Test tubes (3 tubes with extracts
Surname Code Name District / city Workplace Code Total: TASKS of the practical round of the regional stage of the XXVIII All-Russian Olympiad for schoolchildren in biology. 2011-12 academic year year. Grade 11 BIOCHEMISTRY. Equipment,
MICROBIOLOGY EXPLANATORY NOTE TO THE WORKING PROGRAM OF THE COURSE "Microbiology", grade 9 "on the basis of the author's program of the elective course" Microbiology "Averchinkova OE Biology. Elective courses... Curative
TASKS MICROBIOLOGY (max. 20 points) Purpose of work: Prepare and analyze drugs from two known fermented milk products... Equipment: Microscopes, burners or alcohol lamps, glass slides,
KINGDOM OF PROKARYOTES SUBMISSION OF BACTERIA Bacteria are classified as prokaryotes. These are the simplest, smallest and most widespread organisms that have existed on earth for more than 2 billion years, but together
System ORGANIC WORLD All organisms existing on Earth are divided into four kingdoms: Drobyanki, Mushrooms, Plants, Animals. Shotguns belong to prokaryotes (pre-nuclear organisms), fungi, plants,
Project topic Research of microorganisms living in a stagnant reservoir of the Butovo forest in Moscow Author (s): Kasaeva Diana, Kasaeva Kristina, Tkachenko Alisa School: GBOU SOSH 1945 Class: Grade 5 Supervisor:
Laboratory work "The structure of the bodies of cap mushrooms" Purpose: to study the structure of the bodies of cap mushrooms Equipment: cap mushrooms (porcini mushroom, russula, champignon, boletus, butter dish, honey mushrooms, etc.), ready-made
FEDERAL STATE EDUCATIONAL INSTITUTION OF HIGHER PROFESSIONAL EDUCATION "OMSK STATE AGRARIAN UNIVERSITY NAMED AFTER PA STOLYPIN" INSTITUTE OF VETERINARY MEDICINE AND BIOTECHNOLOGY
Approved by Deputy Chief State Sanitary Doctor of the Russian Federation - Chief Physician of the Federal Center for State Sanitary and Epidemiological Supervision of the Ministry of Health of Russia E.N.BELYAEV February 20, 2004 N 24FC / 900 Date
Research work Yeast: the exciting life of small mushrooms Author: student 5 "B" class GBOU Gymnasium 1748 "Vertical" Kutaytsev Georgy Valerievich Supervisor: Nosova Elena Vladimirovna,
Method for detecting yeast and mold fungi in food products METHODOLOGICAL RECOMMENDATIONS Methods for detecting yeast and mold fungi in food: Methodical recommendations - Moscow: Federal Center
Topic 4. Bacteria. Mushrooms. Lichens. Part A. 1. The presence of cyanobacteria in the lichen provides 1) the absorption of atmospheric and soil moisture 2) the use of light for the formation of nutrients
BIOLOGY THE SCIENCE OF LIVING NATURE Biology studies: WHAT DOES BIOLOGY STUDY z the structure and vital functions of living organisms; z laws of individual and historical development organisms. 3 ORGANIC SYSTEM
1. Nitrifying bacteria are classified as 1) chemotrophs 2) phototrophs 3) saprotrophs 4) heterotrophs THEME "Photosynthesis" 2. The energy of sunlight is converted into chemical energy in cells 1) phototrophs
MICROBIOLOGY AND GENETICS Task 1. Prepare and analyze preparations from two known fermented milk products. (max. 10 points) Equipment: Microscopes, burners or spirit lamps, glass slides,
Qualification tests in the specialty "Bacteriology" Bank test items to prepare for certification Select one or more correct answers 1. In accordance with the order of the State Committee on Environmental Protection of the Russian Federation, N mandatory
Tasks 3. Single-celled and multicellular organisms. The kingdom of mushrooms 1. What is contained in the black balls at the ends of the long branches of the mukor mushroom? 1) microscopic fruits 2) nutrients 3)
FEDERAL STATE BUDGETARY EDUCATIONAL INSTITUTION OF HIGHER EDUCATION "ORENBURG STATE AGRARIAN UNIVERSITY" Methodical recommendations for independent work learners
Morphology of microorganisms Option 1 1. Gracilicutes are: A. thin-walled bacteria B. thick-walled bacteria C. bacteria with a defective cell wall. D. bacteria with a delicate cell wall. 2.K
Assignment for students: it is necessary to choose the correct answers (can be from one to several). Morphology of microorganisms Option 1 1. Gracilicutes are: A. thin-walled bacteria B. thick-walled
Algae Algae lower plants Algae is an extensive and heterogeneous group of lower plants. Algae are the most numerous and one of the most important photosynthetic organisms for the planet. They meet
Municipal budgetary educational institution Alarskaya average comprehensive school"Considered" Head of the Ministry of Defense Minutes dated 0 y. "Agreed" Deputy Director for Water Resources Management MBOU 0 g "Approved"
1 Basic techniques of working with microorganisms Task 1. Introduction to microbiology. Safety precautions when working with microorganisms Purpose of the task. This task will introduce you to the rules of safe work in
Tasks 2. Cellular structure of organisms 1. What chemical element is part of the vital organic compounds of the cell? 1) fluorine 2) carbon 3) copper 4) potassium 2. As a storage substance
Molds are the influence of physical factors on the growth and development of fungi. Logunov Stepan 7g Project manager Bulycheva M.B. Relevance of research Fungal spores are highly resistant to unfavorable
Test 5 Option 1. Topic "Mushrooms" Tasks with the choice of one correct answer. 1. The main difference between fungi and plants is that they: have a cellular structure, absorb water and mineral salts from the soil,
1. Explanatory note The program is intended for those entering the postgraduate study of the FSBEI HPE BSU in the specialty 03.02.03 - Microbiology The program for the training of highly qualified specialists in the direction
FEDERAL EDUCATION AGENCY
KARACHAEVO-CHERKESS STATE TECHNOLOGICAL ACADEMY
Department of Production Technology of Livestock Products
MICROBIOLOGY
INSTRUCTIONS
to laboratory and practical studies for students
agrarian institute
Cherkessk - 2010
Compiled on the basis of approximate and work programs on the course "Microbiology" in accordance with the State educational standard of higher professional education in the specialty 110305 "Technology of production and processing of agricultural products" and 110201 "Agronomy" (2000).
Discussed at a meeting of the TPPZ department (minutes of 2.07.2009)
Approved by the methodological commission Agrarian Institute(Minutes No. 6 dated 01.01.2001). Published by the decision of the educational and methodological council of the Karachay-Cherkess State Technological Academy (minutes of 01.01.2001)
Compiled by: candidate of biological sciences, associate professor , Candidate of Agricultural Sciences, Associate Professor , assistant
Reviewers: – candidate of biological sciences
Associate Professor of the Department of Agronomy
Associate Professor of the Department "TPPZH"
Editor: Ph.D. Sciences, Associate Professor
Content
Introduction …………………………………………………………………… .. 6
1. Microscopy ………………………………………………………… .. 7
1.1. Light-field microscopy …………………………………………. .7
1.1.1. Microscope device ……………………………………………… 7
2. Work with microorganisms ………………………………………………. eight
2.1. Methods of preparation of preparations ………………………………………………………………………………………… ..... 8
2.1.1. Technique of taking culture for preparations …………………………… 8
2.1.2. The study of living cells of microorganisms by the method of
pressure drop ……………………………………………………… .. 8
2.1.3. Fixed preparations of microorganisms ……………………… 9
The lighting system is located under the stage. The mirror reflects the incident light into the condenser. One side of the mirror is flat, the other is concave. When working with a condenser, a flat mirror must be used. A concave mirror is used when working without a condenser with low magnification lenses. The condenser consists of 2-3 short-focus lenses, collects the rays coming from the mirror and directs them to the object. A condenser is required when working with immersion systems. The condenser has an iris (petal) diaphragm made of steel crescent-shaped plates.
Colored preparations are considered with an almost completely open diaphragm, unstained ones - with a reduced aperture of the diaphragm.
Lens- multi-lens system, on the quality of which the image of the object depends. The outer lens is called the frontal lens and provides magnification. The rest of the lenses perform the function of correcting optical deficiencies.
Lenses are dry and submerged (immersion). When working with dry objectives, there is air between the front objective lens and the object of study. When working with an immersion objective V = 90x, between the cover glass and the objective lenses there is cedar oil, the refractive index of which is close to the refractive index of the glass 1.515 and 1.52, respectively. The lenses have 8x, 40x and 90x magnifications.
Eyepiece serves as a direct continuation of the "lenses" (lenses) of the human eyes.
The eyepiece consists of two lenses - the upper one - the eye and the lower one - collective, enclosed in a metal frame. The purpose of the eyepiece is to enlarge the image that the lens gives. The eyepiece magnification is engraved on its frame. The working magnification of the eyepieces ranges from 4x to 15x.
Eyepieces are different types, and the choice depends on the lens. When working with a microscope for a long time, they use a binocular attachment, as it improves the visibility of the object, reduces the brightness of the image and thereby preserves vision.
Working with microorganisms2.1. Preparation methods
2.1.1. Technique of taking culture for preparations
A small amount of microbial mass is taken from a test tube with a bacteriological needle, burned in a flame. If the culture is liquid, then it is better to use a loop for this, in other cases - a needle.
2.1.2. Study of living cells of microorganisms by the crushed drop method
Living cells of microorganisms are examined by the crushed drop method, the object is pre-stained with vital dyes - vital coloration(dyes: methylene blue, neutral red in concentrations from 0.001 to 0.0001%).
Preparations are microscoped, slightly darkening the field of view; the condenser is slightly lowered, the flow of light is regulated by a concave mirror. Initially, they use a low magnification - an 8x objective, after the edge of the drop is detected, a 40x or immersion (90x) objective is installed.
In the case of the crushed drop method, a drop of tap water is applied to a clean glass slide. A culture is introduced into it and mixed with water. Excess water is removed with filter paper. When using an immersion objective, a drop of cedar oil is applied to a glass slide and microscoped.
2.1.3. Fixed preparations of microorganisms
Fixed preparations presuppose such processing of living cells, which makes it possible to quickly interrupt the course of life processes in an object, preserving its fine structure. As a result of fixation, the cells are firmly attached to the glass and are better stained. Fixation is necessary when working with pathogenic microorganisms (for safety reasons).
Preparation of the smear. A drop of tap water is applied to a clean, fat-free slide. To degrease glasses, a mixture of ethyl alcohol and sulfuric ether in a 1: 1 ratio is used. Using a calcined bacteriological needle, a small amount of microbial mass is taken from a culture tube and added to a drop of water. The drop is carefully smeared with a loop on the glass over an area of about 4 cm2.
If the suspension is thick, it is first diluted with water. For this, a little suspension is taken with a calcined loop and transferred to a drop of water on another glass slide. A suspension of normal density is smeared with a thin layer on glass, then the smear is dried in air at room temperature or with low heat, holding the drug high above the burner flame. Strong heating of the preparation during drying is not recommended to avoid coagulation of proteins, which distorts the structure and shape of cells. The dried preparation is fixed.
Fixation of the smear. It is carried out over the burner flame or using chemical counions. In the first case, the preparation is slowly carried out three to four times with the lower side over the burner flame, in the second case, chromium compounds are used: formalin, osmic acid, acetone. One of the common fixation techniques is the treatment of the drug with 96% alcohol or a mixture of equal volumes of ethyl alcohol and ether (Nikiforov's liquid). For this, the preparations are immersed in the fixing liquid for 10-30 minutes.
Coloring of the preparation. A few drops of dye are applied to the smear. Depending on the type of dye and the purpose of the study, the duration of staining varies from 1 to 5 minutes, in some cases it takes up to 30 minutes. At the end of staining, the preparation is washed with water, water is removed with filter paper, dried in air and microscoped.
There are simple and differentiated painting methods.
At simple any one dye is used for coloring (methylene blue, fuchsin, gentian violet), the whole cell is stained.
At differentiated By staining, individual cell structures are stained with different dyes (Gram stain, spore staining).
Study of the morphology of microorganisms3.1. Cell shape
3.1.1. Bacteria
In shape, all bacteria are divided into spherical (cocci), rod-shaped and crimped.
Globular bacteria - cocci.
1. Micrococci - single spherical cells ( Micrococcus agilis).
2. Diplococci - spherical cocci, connected in pairs. ( Azotobacter chroococcum).
3. Tetracocci- spherical cocci, connected in four.
4.Streptococci- globular bacteria connected in chains (mainly pathogenic, as well as lactic acid bacteria Lactococcus lactis).
5. Sarcinas- globular bacteria, grouped by 8 cells, arise as a result of cell division in three mutually perpendicular planes. Some types of sarcins form large cuboid packets with 4 sarcins on each side. Typical representative Sarcina flava(sarcinum yellow) - the most common representative of the microflora of the air.
All spherical forms of bacteria, with the exception of Streptoctococcus lactis, viewed on fixed and fuchsin-stained preparations.
Rod-shaped bacteria. These include forms that do not form controversy (childbirth Pseudomonas, Achromobacter, Lactobacillus and others) and forming disputes (childbirth Bacillus, Clostridium and etc.).
Spore-forming stick Pseudomonas stutzeri – its cytoplasm is stained evenly.
Spore-forming rods Bacillus mycoides and Bacillus mesentericus. Under the microscope, they appear unevenly colored. Spores do not stain as denser structures. Cells Bacillus mycoides arranged in chains, these are streptobacilli.
Rod-shaped bacteria are viewed on fixed and stained slides.
Curvy shapes
1. Vibrios – slightly curved cells.
2. Spirillae can have one curl in the form of the Russian letter C, two curls in the form of the Latin letter S, or several - in the form of a spiral.
3. Spirochetes - long and thin cells with a lot of curls; the length of the cells exceeds their thickness by 5-200 times.
Vibrios and spirillae are conveniently viewed on a fixed and colored preparation prepared from slurry, previously incubated for several days in a thermostat. Of the many microorganisms on such a preparation, convoluted forms are often found.
You can get acquainted with spirochetes on a fixed stained preparation of dental plaque; preparations for scraping from a carious tooth are especially successful. Dental spirochetes are very thin, hair-like, short (only 2-3 curls).
3.1.2. Actinomycetes
Actinomycetes are radiant fungi. The mycelium of actinomycetes on nutrient media is differentiated: one part of it is immersed in the substrate (substrate mycelium), the other is located above the substrate (aerial mycelium).
Many representatives of actinomycetes produce pigments, therefore their aerial mycelium and especially colonies are colored blue, blue, purple, pink, brown, brown or black. Actinomycetes stain the culture medium in the appropriate colors.
A piece of actinomycete colony is applied to a glass slide together with the medium. With the second glass slide, press this piece tightly to the glass, crush and smear it on the glass. The drug is dried, fixed, dyed, viewed under a microscope, where mycelial unicellular filaments are partially visible.
3.1.3. Yeast
Yeast - unicellular microscopic fungi of various shapes: elliptical, pear-shaped, round, cylindrical. Reproduce vegetatively and sexually.
For laboratory studies, baker's yeast is used. A small piece of yeast mass a few hours before class is placed in warm sugared water and placed in a warm place. A whitish, cloudy liquid is formed. A drop of this liquid is applied to a glass slide, covered with a cover glass, a drop of cedar oil is applied on top and the preparation is viewed with an immersion system. Budding and dividing cells are visible.
3.2. Chemical research methods
3.2.1. Gram staining of cells of microorganisms
This method of differentiation of microbial cells is based on the difference in chemical composition cell membranes. In the cells of some types of microorganisms, an alcohol-insoluble compound of iodine with the main dye is formed, while in other species this compound appears temporarily and dissolves after treatment with alcohol. Microorganisms of the first group are called gram-positive second - gram-negative.
Gram staining technique. Three thin smears of different cultures of microorganisms are applied to a defatted glass slide (two of them are control ones, with a known relation to Gram stain). The smears are dried in air, fixed over a burner flame and stained for 1 min with a phenolic solution of gentian violet (or crystal violet), holding the glass in a slightly inclined position. Then the dye is poured off and, without rinsing the preparation with water, Lugol's solution is applied to it for 1 min (until the smear is completely blackened). The glass is held in an inclined position. The drug, without rinsing with water, is treated, shaking continuously, with 96% alcohol for 15-20 s. It is important to adhere to the discoloration time, since when the specified period is exceeded, gram-positive cells also discolor.
After washing with water, the preparation is stained with Pfeifer's fuchsin for 1 min. Gram-positive microorganisms acquire a dark purple color, and gram-negative microorganisms are colored in an additional color (fuchsin).
The results of Gram staining depend on the age of the culture: in old cultures, dead cells are always stained with Gram negative. Therefore, it is better to use young one-day crops.
Yeast, Bacillus mesentericus or Bacillus subtilis(gram-positive) and Escherichia coli (gram-negative).
Dyes and reagents for Gram staining.
1. Gentian violet phenolic solution: gentian violet - 1 g, 96% alcohol - 10 ml, crystalline phenol - 2 g, distilled water - 100 ml.
In some cases, apply gentian alcohol solutionpurple: gentian violet (or crystal violet) - 1 g, 96% alcohol (rectified) - 100 ml, glycerin - 5 ml. The mixture is placed in a thermostat for 24 hours, then filtered.
2. Lugol's solution(potassium iodite - 2 g, crystalline iodine - 1 g, distilled water - 300 ml). First, a concentrated solution of potassium iodite in 5 ml of water is prepared, iodine is dissolved in it, then water is added to 300 ml.
3. Alcohol 96%.
4. Fuchsin Pfeifer(an aqueous solution of Tsil's carbolic fuchsin): 1 ml of Tsiel's carbolic fuchsin and 9 ml of distilled water. It is prepared as follows: 1 g of fuchsin, 5 g of crystalline phenol, 96% alcohol - 10 ml, a few drops of glycerin, 100 ml of distilled water, fuchsin is dissolved in ethanol, phenol dissolved in water is added. The solution is stirred and left for several days. It is filtered before use.
3.2.2. Spore coloration in bacteria
Spores of bacteria, in comparison with vegetative cells, are highly resistant to adverse environmental conditions. They are round, oval or elliptical formations. If the diameter of the spore does not exceed the diameter of the cell in which the spore is formed, the cell is called bacillary, if it exceeds, then, depending on the location of the spore in the center or at the end of the cell, this cell is called accordingly clostridial or plectridial . In the bacillary cell, the spore can be located in the center of the cell - central position, at the end - terminal and closer to one end - subterminal position.
When observing living spore-forming bacteria, their spores can be distinguished by the stronger refraction of light rays. Spores are acid-resistant, so they are difficult to stain with dyes. This is explained by the high density of the membrane, the low concentration of free water in it and the high lipid content in the spores. In preparations colored in simple ways or according to Gram, spores remain colorless (negative coloring).
All spore coloring methods are based on common principle: first, the spores are etched with various substances: chromic, hydrochloric, sulfuric, acetic acids, ammonia, caustic soda or hydrogen peroxide, then the cell with the spore is stained when heated and, finally, the cytoplasm is discolored and additionally stained with a contrast dye.
Ziehl-Nielsen method modified by Müller. Before fixing the smear of bacteria on the flame, the preparation is prepared in the usual way. Next, a 5% solution of chromic acid is applied to the cooled and fixed in a flame. After 5-10 minutes, it is washed off with water. The preparation is covered with a strip of filter paper and the paper is abundantly moistened with Tsil's carbolic fuchsin. Heat the preparation over the flame until vapors appear (not to boil), then take it aside and add a new portion of the dye. This procedure is carried out for 7 minutes. It is important that the dye evaporates, but that the paper does not dry out. After cooling, it is removed, the preparation is washed with water and thoroughly blotted with filter paper. As a result of this treatment, cells with spores are evenly stained.
Next, the cytoplasm of the cells (but not the spores) is discolored by treating with a 1% solution of hydrochloric or sulfuric acids for 15-30 s. When preparing a preparation, spores Bacillus mycoides or Bacillus mesentericus it is recommended to discolor the cytoplasm for 16-18 s (measured out loud from 21 to 37-40). If this time is exceeded, spores may also become discolored. Then the preparation is washed with water and stained with methylene blue for 2 min.
The color is contrasting and the bright red spores stand out clearly against the blue background of the cytoplasm.
Peshkov's method. Leffler's methylene blue is poured onto the preparation fixed in the flame, brought to a boil and boiled for 15-20 s, holding the glass over the flame. The smear is washed with water and stained for 30 s with a 0.5% aqueous solution of neutral red. Rinse again, dry and then examine the preparation with oil immersion of the objective. Spores are colored blue or blue, cytoplasm - pink.
For the study of disputes, convenient objects can be Bacillus mesentericus or Bacillus mycoides at the age of 4 days.
Reagents for staining bacterial spores. 1. Carbolic fuchsinTsilya(see 3.2.1).
2. Leffler's Methylene Blue(see 3. 2. .1).
3. Saturated aqueous solution of methylene blue. 2 g of dye and 100 ml of distilled water.
4. Chromic acid, 5% solution.
5. Salt(or sulphuric acid, 1% solution.
4. Cultivation of microorganisms
4.1. Culture media
4.1.1. Preparation of culture media
Meat-peptone broth (BCH). For the preparation of meat-peptone media use meat broth, which is obtained as follows: 500 g of finely chopped fresh meat is poured into an enamel pan with 1 liter of tap water heated to 50 ° C, and left to infuse for 12 hours at room temperature or 1 hour at 50-55 ° C. The meat is wrung out, the extract is filtered through cheesecloth with a layer of cotton wool, boiled for 30 minutes to coagulate colloidal proteins and filtered twice (the first time through cheesecloth with cotton wool, the second through a paper filter). The filtrate is added with water to 1 l, poured into flasks, closed with cotton stoppers and sterilized at 120 ° C for 20 minutes (the flask stoppers are closed on top with paper caps).
Meat broth can be used at any time to prepare media. If they are prepared immediately, then pre-sterilization of the meat broth is not required.
To prepare BCH, add 5-10 g to 1 liter of meat broth peptone(the first product of protein hydrolysis) to increase the caloric content of the medium and 5 g table salt to create osmotic activity. The medium is heated until the peptone dissolves, stirring constantly.
Establish a neutral or slightly alkaline reaction of the medium by adding a 20% Na2C03 solution until the wet red litmus paper turns blue. It is convenient to use an indicator to check the pH of the medium. bromothymallblow: 1-2 drops of it are mixed in a porcelain cup with a drop of broth. In a neutral medium, bromothymolblau is bottle green, in an acidic medium it is yellow, and in an alkaline medium it is blue.
After the pH is established, the medium is boiled again for 5-10 minutes, and the proteins that coagulate when the reaction of the medium changes is filtered through a paper filter without clarifying the broth or clarifying it with protein. For this, fresh egg white is whipped with double the volume of water and mixed with broth cooled to 50 ° C. The mixture is boiled, stirring, over low heat for 10 minutes, then filtered. Transparent meat-peptone broth is poured into test tubes, closed with cotton plugs and sterilized at 120 ° C for 20 minutes.
Meat Peptone Agar (MPA). 15-20 g of agar is added to 1 l of MPB. The medium is heated until the agar dissolves (its melting temperature is 100 ° C, its solidification is 40 ° C), a slightly alkaline reaction of the medium is established with a 20% Na2C03 solution and poured through a funnel into test tubes (approximately 10 ml of agar in a column for subsequent pouring into Petri dishes and 5 ml each to obtain slant agar - stocks).
When spilling agar, the edges of the tubes must remain dry, otherwise the corks will stick to the glass. The tubes with the medium are sterilized in an autoclave at 120 ° C for 20 minutes.
4.2. Sterilization methods
Sterilization - This is the complete destruction of cells of microorganisms in nutrient media, dishes, etc.
Several methods of sterilization are known. Heat sterilization is more commonly used.
4.2.1. Flambing, or calcining
You can ignite platinum loops, needles, spatulas, small metal objects (scissors, lancets, tweezers), as well as glass rods, microscope slides, cover slips, etc. immediately before use.
4.2.2. Dry heat sterilization
It is used for processing dishes and dry materials, such as starch, chalk. In this case, the sterilized object is kept at 170 ° C for 2 hours (from the moment the required temperature is established) in electric drying cabinets. It is not recommended to raise the temperature above 170 ° С: cotton plugs and paper begin to deteriorate.
Before sterilization, glassware is sealed with cotton plugs and wrapped in paper. Cups, test tubes, pipettes, cotton wool, gauze are wrapped in paper or placed in special cases and cases, in which sterile dishes can be stored after sterilization.
At the end of sterilization, the cabinet is opened only after the temperature has dropped to room temperature, otherwise the glass may break.
4.2.3. Sterilization with flowing steam
Flowing steam (100 ° C) is used to treat objects that deteriorate from dry heat, and some nutrient media that cannot withstand higher temperatures (media with carbohydrates, MPF, milk). Sterilization is carried out in a Koch boiler for 30 minutes for 3 days every day. This sterilization is called fractional.
With a single heating at a temperature of 100 ° C for 30 minutes, vegetative cells die, while the spores of many microorganisms remain viable. After such heating, the medium is placed in a thermostat at 28-30 ° C for 24 hours. The spores preserved during the first heating have time to germinate into vegetative forms, which die during subsequent heating. Then this operation is repeated 2 more times.
4.2.4. Sterilization with saturated steam under pressure
This is the fastest and most reliable method of sterilization and kills the most persistent spores. With its help, most culture media and dishes are sterilized.
Treatment with saturated steam is carried out in a hermetically sealed thick-walled boiler - autoclave. On the lid or on the side of the autoclave there is a steam outlet valve, a pressure gauge and a safety valve. The pressure gauge shows how much the steam pressure inside the boiler is higher than normal. To prevent an explosion when the pressure limit is exceeded, a safety valve is activated, releasing steam.
The indicator of the pressure gauge in physical atmospheres corresponds to a certain temperature.
Reliable sterilization is achieved by heating at 120 ° C and a pressure of 1 atm for 20 minutes.
Sterilization is carried out as follows. Water is poured into the autoclave, sterilized items are placed in it, the autoclave lid is screwed on and heating is started. The valve is left open until all the air in the autoclave has been displaced by water vapor. When steam begins to leave the tap in a continuous stream, the tap is closed, the steam pressure in the autoclave is brought to 1 atm and maintained at this level for 20-30 minutes. Then the heating is stopped, they wait until the pressure gauge needle drops to 0, carefully (little by little) open the tap and let off steam. Only then unscrew the lid of the autoclave. If the tap is opened before the pressure drops, then the liquid in the sterilized vessels will boil and push the plugs out of them.
The autoclave is also used for fractional sterilization with flowing steam. In this case, the lid is not screwed on in order to allow the steam to escape freely.
4.2.5. Pasteurization
Pasteurization is incomplete, or partial, sterilization, which means heating at 65-80 ° C for 30-10 minutes, respectively, followed by rapid cooling to 10-11 ° C. Milk, beer, wine and other products are pasteurized.
Materials and equipment
MPB, agar, red litmus, bromothymolblow, porcelain plates with wells or cups, glass rods, 20% Na2C03 solution, test tubes in racks (for pouring agar), funnels, cotton wool, Petri dishes, 1 ml Mohr pipettes, paper for cup and pipette wraps, 250 ml flasks, harsh threads.
5. Counting and isolation of pure culture of microorganisms
5.1. Methods for counting the number of microorganisms
5.1.1. Counting the number of microorganisms (CFU) in soil by the method of nutrient plates in combination with the method of successive dilutions
Soil is the most favorable environment for the development of microorganisms. Due to the high heterogeneity of its composition, to account for the number of microorganisms in it, they take average soil sample.
First, suspensions are prepared (by dilution method) containing different concentrations of soil in 1 ml of water. To do this, a sample of soil in 1 g is taken from a jar or bag onto a sterile watch glass with a sterile porcelain spatula or an aluminum teaspoon. The watch glass, spatula, spoon are flamed in the flame of a burner or moistened with alcohol and burned. When weighing the soil, cover the watch glass with another sterile watch glass.
A sample of soil, observing the conditions of asepsis, is transferred into a 250 ml flask with 99 ml of sterile water. The mixture is shaken for 5 minutes without wetting the cork. Using a sterile pipette, take 1 ml of a suspension containing 10-2 g of soil, and transfer it into a test tube with 9 ml of sterile tap water. The pipette is repeatedly washed with water in a test tube to rinse the cells from its walls as much as possible. With another sterile pipette, take another 1 ml of suspension from the flask and place it in a second flask, also containing 99 ml of sterile tap water. This pipette is washed in the same manner as in the first case. The tube and the second flask are shaken for 1 min. The concentration of soil in the test tube will be 10-3 g, in the second flask -10-4 g. In the same way, 1 ml of suspension from the second flask is transferred with new sterile pipettes into the second test tube with 9 ml and into the third flask with 99 ml of sterile tap water and prepare new suspensions containing 1 ml, respectively, 10-5 and 10-6 g of soil.
PRACTICAL WORKS
For second year students
ON MICROBIOLOGY
1. Microbiological laboratory.
2. Microscopic research methods. Acquaintance with the microscope.
3. Methods for staining preparations. Preparation methods
for microscopy.
4. Methods for collecting material. Culture media.
5. Bacteriological research method on the example of staphylococci.
6. Cultivation and indication of viruses.
9. The influence of environmental factors on microorganisms.
10. Serological tests. Agglutination reaction.
11. Reaction of hemagglutination.
12. Reaction of binding complement.
13. Reaction of precipitation. Ring precipitation reaction.
14. Reaction of immunofluorescence. Immunity reaction (skin tests).
Opsonophagocytic reaction.
15. Vaccines.
16. Serum preparations.
Introduction
Toolkit developed to help students of the Moscow Medical College of Railway Transport MIIT to prepare practical work within the discipline "Fundamentals of Microbiology, Virology and Immunology".
Learning module
Lesson topic: see table of contents
Lesson type: practical
Place of the lesson: laboratory of microbiology
Time: 16 * 2 hours
The student should know:
SECTION 1
FUNDAMENTALS OF MEDICAL
BACTERIOLOGY AND MICROBIOLOGY
PRACTICAL WORK No. 1
Theme:"Microbiological laboratory"
A microbiological laboratory is organized at hospitals and sanitary-epidemiological stations. There are also special laboratories in which they work with pathogens of especially dangerous infections, these are
virological laboratories, etc.
The tasks of the microbiological laboratory include:
1) bacteriological diagnostics of infectious diseases;
2) sanitary and bacteriological studies of water, air and objects
the environment;
The laboratory includes:
1) laboratory room with a glazed box for conducting
microbiological studies (in the box must be installed
bactericidal lamp);
2) washing;
3) preparative (room for the preparation of dishes and other
auxiliary work);
4) autoclave;
5) a room for receiving analyzes and issuing research results;
6) vivarium;
The microbiological laboratory should be equipped with:
- thermostat,
- refrigerator,
- drying chambers,
- centrifuge,
- laboratory glassware;
As well as equipment:
- alcohol or gas burner
- bacteriological loops
- des. solution